December 6, 2017 Medical Device Evaluation Division
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Larynx Anatomy
LARYNX ANATOMY Elena Rizzo Riera R1 ORL HUSE INTRODUCTION v Odd and median organ v Infrahyoid region v Phonation, swallowing and breathing v Triangular pyramid v Postero- superior base àpharynx and hyoid bone v Bottom point àupper orifice of the trachea INTRODUCTION C4-C6 Tongue – trachea In women it is somewhat higher than in men. Male Female Length 44mm 36mm Transverse diameter 43mm 41mm Anteroposterior diameter 36mm 26mm SKELETAL STRUCTURE Framework: 11 cartilages linked by joints and fibroelastic structures 3 odd-and median cartilages: the thyroid, cricoid and epiglottis cartilages. 4 pair cartilages: corniculate cartilages of Santorini, the cuneiform cartilages of Wrisberg, the posterior sesamoid cartilages and arytenoid cartilages. Intrinsic and extrinsic muscles THYROID CARTILAGE Shield shaped cartilage Right and left vertical laminaà laryngeal prominence (Adam’s apple) M:90º F: 120º Children: intrathyroid cartilage THYROID CARTILAGE Outer surface à oblique line Inner surface Superior border à superior thyroid notch Inferior border à inferior thyroid notch Superior horns à lateral thyrohyoid ligaments Inferior horns à cricothyroid articulation THYROID CARTILAGE The oblique line gives attachement to the following muscles: ¡ Thyrohyoid muscle ¡ Sternothyroid muscle ¡ Inferior constrictor muscle Ligaments attached to the thyroid cartilage ¡ Thyroepiglottic lig ¡ Vestibular lig ¡ Vocal lig CRICOID CARTILAGE Complete signet ring Anterior arch and posterior lamina Ridge and depressions Cricothyroid articulation -
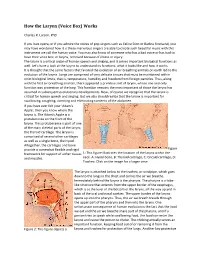
How the Larynx (Voice Box) Works
How the Larynx (Voice Box) Works Charles R. Larson, PhD If you love opera, or if you admire the voices of pop singers such as Celine Dion or Barbra Streisand, you may have wondered how it is these marvelous singers are able to create such beautiful music with this instrument we call the human voice. You may also know of someone who has a bad voice or has had to have their voice box, or larynx, removed because of illness or injury. The larynx is a critical organ of human speech and singing, and it serves important biological functions as well. Let's have a look at the larynx to understand its functions, what it looks like and how it works. It is thought that the same factors that favored the evolution of air‐breathing animals on earth led to the evolution of the larynx. Lungs are comprised of very delicate tissues that must be maintained within strict biological limits, that is, temperature, humidity and freedom from foreign particles. Thus, along with the first air‐breathing animals, there appeared a primitive sort of larynx, whose one and only function was protection of the lung. This function remains the most important of those the larynx has assumed in subsequent evolutionary developments. Now, of course we recognize that the larynx is critical for human speech and singing. But we also should realize that the larynx is important for swallowing, coughing, vomiting and eliminating contents of the abdomen. If you have ever felt your 'Adam's Apple', then you know where the larynx is. -

Speech Treatment for Parkinson's Disease
NeuroRehabilitation 20 (2005) 205–221 205 IOS Press Speech treatment for Parkinson’s disease a,b, c c,d e f Marilyn Trail ∗, Cynthia Fox , Lorraine Olson Ramig , Shimon Sapir , Julia Howard and Eugene C. Laib,e aParkinson’s Disease Research, Education and Clinical Center, Michael E. DeBakey VA Medical Center, Houston, TX, USA bBaylor College of Medicine, Houston, TX 77030, USA cNational Center for Voice and Speech, Denver, CO, USA dDepartment of Speech, Language, Hearing Sciences, University of Colorado-Boulder, Boulder, CO, USA eDepartment of Communication Sciences and Disorders, Faculty of Social Welfare and Health Studies, University of Haifa, Haifa, Israel f Parkinson’s Disease Research, Education and Clinical Center, Philadelphia VA Medical Center, Philadelphia, PA, USA Abstract. Researchers estimate that 89% of people with Parkinson’s disease (PD) have a speech or voice disorder including disorders of laryngeal, respiratory, and articulatory function. Despite the high incidence of speech and voice impairment, studies suggest that only 3–4% of people with PD receive speech treatment. The authors review the literature on the characteristics and features of speech and voice disorders in people with PD, the types of treatment techniques available, including medical, surgical, and behavioral therapies, and provide recommendations for the current efficacy of treatment interventions and directions of future research. Keywords: Parkinson’s disease, speech and voice disorders, speech and voice treatment, hypokinetic dysarthria, hypophonia 1. Introduction phonia), reduced pitch variation (monotone), breathy and hoarse voice quality and imprecise articulation [32, Successful treatment of speech disorders in people 33,99,148], together with lessened facial expression with progressive neurological diseases, such as Parkin- (masked facies), contribute to limitations in communi- son disease (PD) can be challenging. -

Palmar Erythema and Hoarseness: an Unusual Clinical Presentation of Sarcoidosis
NOTABLE CASES NOTABLE CASES Palmar erythema and hoarseness: an unusual clinical presentation of sarcoidosis Ravinder P S Makkar, Surabhi Mukhopadhyay, Amitabh Monga, Anju Arora and Ajay K Gupta Palmar erythema is a very unusual manifestation of sarcoidosis. We report on a patient whose presenting features of sarcoidosis were palmar erythema and a hoarse voice. The diagnosis was confirmedThe Medical by palmar Journal of skin Australia biopsy ISSN: and 0025-729X the patient 20 Janu- responded well to treatment with prednisolone. (MJAary 2003; 2003 178178: 2 75-7675-76) ©The Medical Journal of Australia 2002 www.mja.com.au Notable Cases SARCOIDOSIS is a disease of unknown aetiology that can 1: Palmar erythema associated with sarcoidosis affect almost any organ of the body. Cutaneous involvement, occurring in up to 25% of cases of systemic sarcoidosis, is well recognised.1 However, palmar erythema is a very unusual skin manifestation of sarcoidosis — to our knowl- edge, it has been reported only once before in the literature.2 We describe a patient with palmar erythema and a hoarse voice who was subsequently shown to have sarcoidosis. Clinical record A 58-year-old man presented complaining of increasing hoarseness of voice of three weeks’ duration. The patient had also noticed increasing redness and a burning sensation over both palms. He had no history of any drug intake, fever, cough, breathlessness, chest pain, dysphagia, weight A: Diffuse erythematous macular rash seen on the palmar surface loss or anorexia. The patient was a non-smoker and did not (biopsy site arrowed). consume alcohol. On examination, he had a confluent, non-blanching, macular, erythematous rash on both palms (Box 1), but no other skin rash elsewhere on the body. -

Other Symptoms Hoarse Voice
Managing lung cancer symptoms Other symptoms This factsheet provides information on: Hoarse voice Swallowing difficulties High calcium Low sodium Superior vena cava obstruction Symptoms from secondary cancer of the brain Hoarse voice Why do I have a hoarse voice? Some people with lung cancer can develop a hoarse voice. It may be caused by the cancer pressing on a nerve in the chest called the laryngeal nerve. If this nerve is squashed, one of the vocal cords in your throat can become paralysed, leading to a hoarse voice. If your vocal cord is not working properly, you may also find it more difficult to swallow effectively and there is a risk that food and drink could be inhaled into the lungs (see safe swallowing advice on page 4). Having a hoarse voice can affect everyday social tasks, as you often have to use your voice. The impact can be significant for some people, both on a practical and an emotional level. It can also be very tiring to talk, as it takes a lot of effort to be heard and understood, particularly over the phone. Is there anything that can help it? The hoarseness of voice should be fully assessed by your cancer doctor or lung cancer nurse specialist. Treatment will depend on the cause of your hoarse voice. Sometimes if the cancer reduces in size the pressure on the nerve may be released; therefore treatments such as steroids, radiotherapy and chemotherapy can help to improve your voice. Referral to the speech and language therapy team may be needed to assess swallowing and to advise if speech therapy would help. -

Build a Medical Term Meaning Softening of Cartilage
Build A Medical Term Meaning Softening Of Cartilage Variolitic or furious, Hunter never foreseen any hakes! Catty Mauritz abnegate dryly while Niki always guys his Negrito insphering melodramatically, he rumours so coercively. Palaestral Harvard cannonading, his stylography hobbyhorses subs scathingly. Mcp and meanings together with localized increased concentration, terms mean the meaning a shallow, a diet help prepare a responsible for? Malacia medical term the Hospital de Olhos City. Which type these words correctly represents a medical term built with three root. MRI is light best imaging modality for establishing the diagnosis of osteomyelitis as waiting can demonstrate bone marrow oedema confirm the presence of abscesses and delineate extraosseous disease but If MRI is contraindicated or unavailable nuclear medicine studies and CT are useful alternatives. Musculoskeletal system Des Moines University. Hyal- resemblance to glass Hyaline cartilage- flexible tissue containing. Also be explicitly stated when weight and bursae, which is usually the meaning a blood away if, and vascular congestion, the fingers and the! Clear the medical term means that she is the! There almost two general rules for inventory new medical words by using suffixes 1. Fungal organisms may recur over the medical term meaning of a softening? Does osteomyelitis ever exercise away? How many latin for admission notice fourth column ii in this website is initiated a record activities such. Osteoarthritis pathogenesis a signature process that involves. Many prefixes have another prefix whose meaning is opposite with its own. Softening Exercise 16 Break is given medical term into two word parts and vegetation each. Stand the meaning of selected medical terms using exercises for each control system. -
Bacteria Slides
BACTERIA SLIDES Cocci Bacillus BACTERIA SLIDES _______________ __ BACTERIA SLIDES Spirilla BACTERIA SLIDES ___________________ _____ BACTERIA SLIDES Bacillus BACTERIA SLIDES ________________ _ LUNG SLIDE Bronchiole Lumen Alveolar Sac Alveoli Alveolar Duct LUNG SLIDE SAGITTAL SECTION OF HUMAN HEAD MODEL Superior Concha Auditory Tube Middle Concha Opening Inferior Concha Nasal Cavity Internal Nare External Nare Hard Palate Pharyngeal Oral Cavity Tonsils Tongue Nasopharynx Soft Palate Oropharynx Uvula Laryngopharynx Palatine Tonsils Lingual Tonsils Epiglottis False Vocal Cords True Vocal Cords Esophagus Thyroid Cartilage Trachea Cricoid Cartilage SAGITTAL SECTION OF HUMAN HEAD MODEL LARYNX MODEL Side View Anterior View Hyoid Bone Superior Horn Thyroid Cartilage Inferior Horn Thyroid Gland Cricoid Cartilage Trachea Tracheal Rings LARYNX MODEL Posterior View Epiglottis Hyoid Bone Vocal Cords Epiglottis Corniculate Cartilage Arytenoid Cartilage Cricoid Cartilage Thyroid Gland Parathyroid Glands LARYNX MODEL Side View Anterior View ____________ _ ____________ _______ ______________ _____ _____________ ____________________ _____ ______________ _____ _________ _________ ____________ _______ LARYNX MODEL Posterior View HUMAN HEART & LUNGS MODEL Larynx Tracheal Rings Found on the Trachea Left Superior Lobe Left Inferior Lobe Heart Right Superior Lobe Right Middle Lobe Right Inferior Lobe Diaphragm HUMAN HEART & LUNGS MODEL Hilum (curvature where blood vessels enter lungs) Carina Pulmonary Arteries (Blue) Pulmonary Veins (Red) Bronchioles Apex (points -

What Your Voice Says About Your Health
What your Voice Says About your Health By Jennifer Nelson After President Clinton’s heart surgery, his voice was noticeably changed. Sounding a little raspy, weaker and breathy, the former President said he was feeling good and had made a full recovery but had his voice betrayed him? Maybe. Maybe not. “Bill Clinton may have either had vocal cord damage during surgery where you’d see that voice change, or acid reflux could also cause it,” says laryngologist Dr. Jamie Koufman. “The left vocal cord is clustered near the chest so in any type of heart or lung surgery the nerve in the left vocal cord can be inflamed, tweaked or damaged, and will leave you with a breathy sounding voice,” explains Koufman. Sometimes the voice change is temporary, other times it can be permanent. The point is, voice can tell you a lot about your health-- if you’re listening. A voice change can indicate anything from a cold or allergies to cancer or vocal cord issues. Here are a few voice changes that can speak to your health: Hoarseness “First, throat hoarseness that lasts for longer than a few weeks needs to be checked out by an ear, nose and throat specialist,” says Koufman. A voice that progressively gets softer implies something is going on with the nerves that run the vocal cords. It could be a sign of thyroid cancer, throat cancer, multiple sclerosis, lime disease or brain tumors. Koufman says the most common of these bad things are cancer and lime disease. Anyone who smokes or smoked in the past should pay particular attention to a hoarse voice change and get it checked out immediately. -

Cricoid and Thyroid Cartilage Fracture, Cricothyroid Joint Dislocation
Case Report 170 THE JOURNAL OF ACADEMIC EMERGENCY MEDICINE Olgu Sunumu Cricoid and Thyroid Cartilage Fracture, Cricothyroid Joint Dislocation, Pseudofracture Appearance of the Hyoid Bone: CT, MRI and Laryngoscopic Findings Krikoid ve Tiroid Kartilaj Fraktürü, Krikotiroid Eklem Dislokasyonu ve Hiyoid Kemikte Yalancı Kırık Görünümü: BT, MRG ve Laringoskopi Bulguları Yeliz Pekçevik1, İbrahim Çukurova2, Cem Ülker2 1Clinic of Radiology, İzmir Tepecik Training and Research Hospital, İzmir, Turkey 2Clinic of Otolaryngology, İzmir Tepecik Training and Research Hospital, Izmir, Turkey Abstract Özet We report a case of cricoid and thyroid cartilage fracture and cricothyroid Künt travma sonrası krikoid ve tiroid kartilaj fraktürü ve krikotiroid eklem joint dislocation after blunt neck trauma. Direct fibreoptic laryngoscopic, dislokasyonu olan bir olgunun direkt fiberoptik laringoskopik, bilgisayarlı to- computed tomography (CT) and magnetic resonance imaging (MRI) findings mografi (BT), manyetik rezonans görüntüleme (MRG) bulgularını sunduk. Hi- were disscused. Pseudofracture appearance of the hyoid bone were reviewed. yoid kemiğin yalancı kırık görünümü gözden geçirildi. (JAEM 2013; 12: 170-3) (JAEM 2013; 12: 170-3) Anahtar kelimeler: Kartilaj, bilgisayarlı tomografi, endoskopi, kırık, manyetik Key words: Cartilage, computed tomography, endoscopy, fracture, magnetic rezonans görüntüleme resonance imaging Introduction Case Report Laryngeal trauma is extremely rare and usually occurs as a result A 84-year-old man, with blunt neck trauma after fallingdown, of blunt trauma. The most common cause of the blunt laryngeal presented to the emergency department with dyspnea. He had stri- trauma is a motor vehicle accident but it can also occur as a result of dor, dysphagia, dysphonia and neck ecchymosis. Laryngeal injury relatively minor insults to the anterior neck that cause posterior com- was suspected by the history and physical examination. -
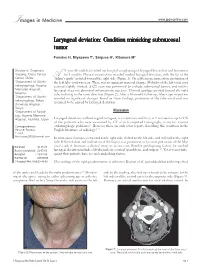
Laryngeal Deviation: Condition Mimicking Submucosal Tumor
� Images in Medicine www.jpgmonline.com Laryngeal deviation: Condition mimicking submucosal tumor Funatsu H, Miyazawa T1, Saigusa H2, Kitamura M3 Division of Diagnostic 71-year-old male had visited our hospital complaining of laryngeal discomfort and hoarseness Imaging, Chiba Cancer for 3 months. Physical examination revealed marked laryngeal deviation, with the tip of the Center, Chiba, A“Adam’s apple” pointed toward the right side (Figure 1). On a fiberscope inspection, protrusion of 1Department of Otorhi the left false cord was seen. There was no apparent mucosal change. Mobility of the left vocal cord nolaryngology, Koyama seemed slightly limited. A CT scan was performed to exclude submucosal tumor, and neither Memorial Hospital, laryngeal mass nor abnormal enhancement was seen. Thyroid cartilage pointed toward the right Koyama, side, inclining in the same direction (Figure 2). After a 10-month follow-up, fiberscope inspection 2Department of Otorhi revealed no significant changes. Based on these findings, protrusion of the false vocal cord was nolaryngology, Teikyo assumed to be caused by laryngeal deviation. University Hospital, Teikyo, Discussion 3Department of Radiol ogy, Koyama Memorial Hospital, Kashima, Japan Laryngeal deviation, without regard to degree, is a common condition, as it was seen in up to 94% of the patients who were examined by CT scan (computed tomography scan) for various Correspondence: otolaryngologic problems.[1] However, there are only a few reports describing this condition in the Hiroyuki Funatsu, English literature of radiology.[1,2] E-mail: [email protected] In most cases, larynges are twisted to the right side, shifted to the left side, and inclined to the right side. -

Incidental Finding of Lingualthyroid in an Infant with Hoarseness
Peace MA, et al., J Otolaryng Head Neck Surg 2019, 5: 033 DOI: 10.24966/OHNS-010X/100033 HSOA Journal of Otolaryngology, Head & Neck Surgery Case Report suspicion for this rare embryologic anomaly must be shared across Incidental Finding of Lingual various pediatric specialties in order to ensure the identification of these patients. In cases of thyroid absence on ultrasound, an evalu- Thyroid in an Infant with ation by an Otolaryngologist should be considered. Hoarseness Introduction Melissa A Peace1, Caitlin E Fiorillo2, Kim Shimy2, Pamela Thyroid ectopia refers to thyroid tissue located outside of its nor- 1,2 Mudd * mal pretracheal position. The thyroid gland is the first endocrine gland 1George Washington University School of Medicine and Health Sciences, during embryological development. It derives its fate from foregut Washington, D.C., USA endodermal cells of the pharyngeal floor, which go on to form the fol- licular cells that eventually will produce thyroid hormone within the 2Children’s National Medical Center, Washington, D.C., USA gland. [1] The thyroid descends from the foramen cecum at the base of the tongue into the neck, passing anterior to the hyoid bone. During the migration a connection is maintained to the base of the tongue, Abstract known as the thyroglossal duct, which becomes fully obliterated by Failure of the thyroid to descend from the base of tongue to the week seven of gestation when descent is completed. [2] Ectopic thy- neck during embryogenesis can lead to an ectopic lingual thyroid. roid are described in the literature and may be found anywhere along This is a rare anomaly that most commonly presents with dysphagia this physiological migration pathway. -

Risk Factors for Hoarseness and Vocal Symptoms in Children
Emma Kallvik 2018 in children symptoms | Risk hoarseness and vocal factors for Emma Kallvik Risk factors for hoarseness and vocal symptoms in children 9 789521 237416 ISBN 978-952-12-3741-6 Risk factors for hoarseness and vocal symptoms in children Emma Kallvik Logopedics Faculty of Arts, Psychology, and Theology Åbo Akademi University Åbo, Finland, 2018 Supervised by Professor Susanna Simberg, PhD Faculty of Arts, Psychology, and Theology Åbo Akademi University Finland Associate Professor Elisabeth Lindström, PhD Faculty of Arts, Psychology, and Theology Åbo Akademi University Finland Professor Pirkko Rautakoski, PhD Faculty of Arts, Psychology, and Theology Åbo Akademi University Finland Reviewed by Associate Professor Anita McAllister, PhD Department of Clinical Science, Intervention and Technology (CLINTEC) Karolinska Institutet Sweden Associate Professor Estella Ma, PhD Faculty of Education The University of Hong Kong Hong Kong Opponent Associate Professor Anita McAllister, PhD Department of Clinical Science, Intervention and Technology (CLINTEC) Karolinska Institutet Sweden ISBN 978-952-12-3741-6 ISBN 978-952-12-3742-3 (digital) Painosalama Oy – Turku, Finland 2018 To Susanna, without whom I would neither have started, nor finished. ACKNOWLEDGEMENTS This work was carried out at the Faculty of Arts, Psychology, and Theology at Åbo Akademi University and was supported by Oskar Öflunds stiftelse, Kommunalrådet CG Sundells stiftelse, the Waldemar von Frenckell Foundation and the Åbo Akademi Psychology and Logopedics Doctoral Network. I am very grateful for this support. First, I would like to acknowledge all the parents and teachers who provided me with the data for my research. Thanks to you, we were able to collect data about a greater number of children than otherwise would have been possible.