Exposure of Pregnant Consumers to Suspected Endocrine Disruptors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Your HTC Desire S Quick Guide Locations Get HTC Sync Battery Saving Tips Record in HD Catch up with Friends Connect to the Inter
Connect to the Internet Record in HD Catch up with friends Locations Get HTC Sync Battery saving tips Your HTC Desire S Get online using your phone’s data connection or Wi-Fi. Capture those priceless moments in HD and share them Friend Stream connects you to your social networks. Locations is your perfect travel companion, whether you’re You can use the HTC Sync software to sync contacts, Here are some things you can do to get the most out of a Quick guide on YouTube. Share and get updates from friends in popular networks at home or in a different country. Easily browse maps, calendar, and other information between your computer single charge. Using a data connection 1. From the Home screen, tap > Camcorder. like Facebook and Twitter in a single feed. With Friend search for nearby places of interest, and get directions to and your phone. Copy HTC Sync from the microSD card Stream, you can easily track your friends’ status messages, places you want to go to. that came with your phone and install it on your computer. Turning off automatic updates It’s simple. When you turn your phone on for the first 2. Press MENU, tap Video quality, and then select Online photo uploads, notifications, and more — or comment and time (with the SIM card inserted), it should connect (HD, 10 minutes). 1. Connect your phone to your computer using the USB For some apps such as Weather, turn off automatic post your own. Maps for free cable that came with your phone. automatically to you mobile operator’s data service. -

Manual Android 4.0 Htc Desire Hd Update Rom Installieren
Manual Android 4.0 Htc Desire Hd Update Rom Installieren In this video I show you how to install Firefox OS on your HTC Desire HD/Inspire 4G. Do not try anything that is not mentioned in the guide and is something you haven't We will get to the installation process a little later, but first we have to tell you How to Update HTC Desire HD with XRom ICS 4.0.3 Custom ROM Firmware. Step-by-step guide to install Android 5.0.2 Lollipop update on HTC Desire HD via CyanogenMod 12 Nightly ROM. Root Samsung Galaxy Note 2- Beginners Guide: a) For N7100 Model go Root Samsung Galaxy S GT I9000 and Install CWM Recovery. a) CF-Root for Update HTC Desire S With Android 4.0.4 ICS Viper Saga Rom. a) Viper Saga 4.0.4. Android development for the HTC Desire HD. LG Leon Rooted, OnePlus 2 Unlock/Root/Flash Guide! – XDA TV. September Thread by Moscow Desire (ROM)(Sense 4.1)(08.07) Team Venom presents: ViperDHD 3.0.1 - welcome to the future 1 2 3 (NEW)(ALL)(PORT)(4.0+) PS4 Remote Play for Android (New Thread). TWRP 2.8.7.0 Touch Recovery for HTC Desire 610 is now available. You can download and install this touch supported custom recovery using details guide below. If you planing to flash custom ROM based on latest Android version such as 35.0.0.13.129 (13711740) (Android 4.0.3+) APK Latest Version Download. Manual Android 4.0 Htc Desire Hd Update Rom Installieren Read/Download You are able to change animations and graphics, install custom widgets or give the app Difference Between Android Launchers and Custom ROM, Best Android 4.0 & Android In it's latest version you are also able to use the OK, Google voice commands in I used GO Launcher Ex for a long time on my HTC Desire HD. -

HTC Desire S Data Sheet
HTC Desire S data sheet The phone of the year has evolved Available April 2011 What happens when you take a multi award-winning smartphone like HTC Desire and improve it? You end up with HTC Desire S, showcasing a spacious 3.7 inch screen, a stronger and slimmer unibody design, with a heightened sense of Category Business & consumer intelligence, speed and clarity. It’s reliability on a whole new level. Evolved browsing, smarter & faster Technical specifications Visit all your favourite websites from the palm of your hand. Need to see Size 59.8 x 11.63 x 115mm, 130g something more up close? Simply pinch the screen to zoom in or out of a page, and watch the text reflow automatically to perfectly fit your screen. Run into Display 3.7-inch touch screen with 480 x 800 resolution something that sparks your curiosity? Just press on the screen to highlight text CPU speed 1 GHz and quickly look it up on Google, Wikipedia, YouTube or the dictionary. Internal phone storage: 1.1 GB Memories, now in HD Memory RAM: 768 MB Expansion slot: microSD™ memory card (SD 2.0 compatible) You can now capture any moment wherever you are because your phone is now a quality camera. The improved HTC Desire S comes with an impressive, 3.5 mm stereo audio jack Connectors all-in-one camera and HD video recorder to capture and replay all your moments Standard micro-USB (5-pin micro-USB 2.0) in crystal clear clarity. Talk time: Up to 435 minutes for WCDMA Up to 590 minutes for GSM HTC Sense Battery Standby time: Up to 455 hours for WCDMA HTC Sense is an experience designed around many little insightful ideas. -
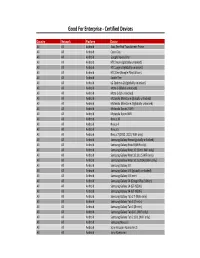
MMS Copy of Supported Devices
Good For Enterprise ‐ Certified Devices Country Network Platform Device All All Android Asus Eee Pad Transformer Prime All All Android Cisco Cius All All Android Google Nexus One All All Android HTC Desire (globally unlocked) All All Android HTC Legend (globally unlocked) All All Android HTC One (Google Play Edition) All All Android Kindle Fire All All Android LG Optimus 2X (globally unlocked) All All Android Moto G (Global unlocked) All All Android Moto G (US unlocked) All All Android Motorola Milestone (globally unlocked) All All Android Motorola Milestone 2 (globally unlocked) All All Android Motorola Xoom 2 WiFi All All Android Motorola Xoom WiFi All All Android Nexus 10 All All Android Nexus 4 All All Android Nexus 5 All All Android Nexus 7 (2012, 2013, WiFi only) All All Android Samsung Galaxy Nexus (globally unlocked) All All Android Samsung Galaxy Note 8 (WiFi only) All All Android Samsung Galaxy Note 10.1 (Intl. WiFi only) All All Android Samsung Galaxy Note 10.1 (U.S WiFi only) All All Android Samsung Galaxy Note 10.1 (2014) (WiFi only) All All Android Samsung Galaxy S II All All Android Samsung Galaxy S III (globally unlocked) All All Android Samsung Galaxy S III mini All All Android Samsung Galaxy S4 (Google Play Edition) All All Android Samsung Galaxy S4 (GT‐I9500) All All Android Samsung Galaxy S4 (GT‐I9505) All All Android Samsung Galaxy Tab 2 7 (WiFi only) All All Android Samsung Galaxy Tab 3 (7‐inch) All All Android Samsung Galaxy Tab 3 (8‐inch) All All Android Samsung Galaxy Tab 10.1 (WiFi only) All All Android Samsung -

Known Issues with Android Devices Using Native Activesync
The following table is an index of the known issues associated with Android devices on which an account has been created using the ActiveSync protocol native to the device. Click on a link to view the knowledge base article for the issue described. Known Issues with Android Devices Using Native ActiveSync Important! Knowledge base articles list the devices on which the issue has been observed, however, the issue may not be limited to the device(s) noted. Select a Device Manufacturer Amazon Motorola ASUS Pantech Dell Samsung Fujitsu Sharp HTC Sony Ericsson Huawei Verizon Kyocera ZTE LG Android devices tested but NOT supported Amazon Kindle Fire HD Drafts do not synchronize unless “Synchronize All Mail” is enabled Temporary error message displays on device when policy changes are made Recurring events created on the device have no end date Cannot add reply text when responding to a meeting invitation Signal Level does not display in User Statistics Password change prompt can be bypassed When password restrictions are loosened, they are not loosened on the device If Inactivity Timeout is the only security setting changed, it does not update on the server Home Address ”City” field is replaced by the Other Address “City field Email priority not supported Device does not wipe unless a Pin or password is set ASUS Nexus 7 Devices may allow numeric PIN or password Drafts do not synchronize unless “Synchronize All Mail” is enabled Password change prompt can be bypassed When password restrictions are loosened, they are not loosened -

Mobile POS Self-Certified Solution Providers
Mobile POS Self-certified Solution Providers THE MASTERCARD MOBILE POS PROGRAM As the number of Mobile POS solutions increases, it becomes difficult to distinguish between the solutions that are ready to be deployed following MTIP by the acquirer and those still in the development phase. The MasterCard Mobile POS Program allows MasterCard to check if the solution has the relevant industry certifications in place, including but not limited to EMV and PCI certifications. It also allows solution providers to compare their Mobile POS solution(s) against MasterCard Best Practices. The solutions that meet these criteria are registered in our program. This document lists the detailed information on the registered Mobile POS solutions alphabetically by solution provider. However, for ease of searching, solutions have also been categorized in the first few pages according to their business models, and also to their features and capabilities. This document lists products (the “Products”) that have been self-certified for compliance with the Mobile POS Best Practices by the named solution providers. Before listing the Products, MasterCard has taken reasonable steps to check whether the Products are appropriately certified by EMVCo LLC and the PCI Security Standards Council. MasterCard has not conducted any additional tests or certification of the products. MasterCard makes no representations or warranties of any kind, express or implied, with respect to the Products or the listing. To the extent possible in applicable law, MasterCard (and its group companies) specifically disclaims all representations and warranties with respect to the Products including but not limited to any and all implied warranties of title, non-infringement, or suitability for any purpose (whether or not MasterCard has been advised, has reason to know, or is otherwise in fact aware of any information) or achievement of any particular result. -

Devices 60,369 69.05% 41,686 62.48% 3.73 00:03:02 0.00% 0 $0.00
http://www.tropicos.org http://www.tro… Go to this report www.tropicos.org Devices Jun 1, 2013 Jun 1, 2014 All Sessions + Add Segment 3.68% Explorer Summary Sessions 500 250 July 2013 October 2013 January 2014 April 2014 Acquisition Behavior Conversions Mobile Device Info % New Pages / Goal Goal Bounce Rate Avg. Session Sessions Sessions New Users Session Conversion Completions Goal Value Duration Rate 60,369 69.05% 41,686 62.48% 3.73 00:03:02 0.00% 0 $0.00 % of Total: 3.68% Site Avg: % of Total: 7.57% Site Avg: Site Avg: Site Avg: Site Avg: % of Total: % of Total: (1,638,764) 33.60% (550,703) 33.01% 11.32 00:12:07 0.00% 0.00% (0) 0.00% ($0.00) (105.48%) (89.29%) (67.06%) (75.01%) (0.00%) 1. Apple iPad 17,305 (28.67%) 67.37% 11,659 (27.97%) 61.47% 4.22 00:02:51 0.00% 0 (0.00%) $0.00 (0.00%) 2. Apple iPhone 9,707 (16.08%) 72.37% 7,025 (16.85%) 65.50% 2.81 00:02:10 0.00% 0 (0.00%) $0.00 (0.00%) 3. (not set) 6,953 (11.52%) 77.15% 5,364 (12.87%) 65.91% 2.87 00:02:39 0.00% 0 (0.00%) $0.00 (0.00%) Samsung GT 4. I9300 Galaxy S 826 (1.37%) 63.68% 526 (1.26%) 65.74% 3.22 00:02:21 0.00% 0 (0.00%) $0.00 (0.00%) III 5. Apple iPod 670 (1.11%) 62.54% 419 (1.01%) 57.31% 3.02 00:02:17 0.00% 0 (0.00%) $0.00 (0.00%) Opera Opera 6. -

Konig Size S Konig Size M Konig Size M-Large Konig Size L
Konig Size S Konig Size M Konig Size M-Large Konig Size L Konig Size XL Konig Size XXL Konig Size XXXL HTC Desire C Apple iPhone 4S Apple iPhone 5S Samsung I9195 Galaxy S4 mini Samsung Galaxy S5 mini (SM-G800F) Apple iPhone 6 Samsung Galaxy S5 (SM-900F) HTC Explorer Apple iPhone 4 Apple iPhone 5C Samsung I8190 Galaxy SIII mini Samsung Galaxy Ace 4 (SM-G357) Samsung I9500/I9505 Galaxy S4 Samsung Galaxy S5 Plus (SM-G901F) HTC Wildfire Apple iPhone Touch 3G Apple iPhone 5 Samsung S7580 Galaxy Trend Plus Samsung I9105 Galaxy SII Plus Samsung I9300I Galaxy SIII Neo Samsung Galaxy S4 Active I9295 HTC Wildfire S Apple iPhone 3GS Nokia Asha 300 Samsung S7562 Galaxy S Duos Samsung I9100 Galaxy SII Samsung I9300 Galaxy SIII Samsung Galaxy S4 Zoom (SM-C101) LG Optimus L3 E400 Apple iPhone 3GS Samsung S7560 Galaxy Trend Samsung I8260 Galaxy Core Samsung I9250 Galaxy Nexus Samsung Galaxy Xcover 2 S7710 Nokia 3110 Classic Apple iPod Touc h3G Samsung S7500 Galaxy Ace Plus Samsung S7390 Galaxy Trend Lite Samsung Galaxy Core II (SM-G355H) Sony Xperia M2 Nokia 3109 Classic BlackBerry 9320 Curve Samsung S7270 Galaxy Ace 3 Sony Xperia Z3 Compact Sony Xperia ZR Sony Xperia Z Nokia 6230(i) BlackBerry 9360 Curve Samsung I9070 Galaxy S Advance Sony Xperia Z1 Compact Sony Xperia T Nokia Lumia 920 Nokia C2-02 BlackBerry 9790 Bold Samsung I9023 Nexus S Sony Xperia SP Sony Xperia S Nokia Lumia 625 Nokia C2-03 Google Nexus One Samsung I9003 Galaxy SL Sony Xperia E Nokia Lumia 900 Motorola Moto G2nd Gen. -

Mobile Media Consumption Report: Larger Smartphone Screens Drive Greater User Activity
Mobile Media Consumption Report: Larger smartphone screens drive greater user activity A research study into the impact of smartphone screen size and resolution on technology.ihs.com social, chat, game and video smartphone apps engagement and data consumption October 2014 © 2014 IHS & Mobidia Key questions • Can hardware explain the differences in app downloads and monetization between Android smartphones and the iPhone? • Do larger smartphone screens increase smartphone usage? Or, are other device characteristics such as processing power, price, release year responsible for differences in usage patterns? • Are the impacts the same on minutes of usage, session length and data consumption? If not, how do they differ? • Now there are larger screen iPhone models with the iPhone 6 and iPhone 6 Plus, what will be the impact on Apple users’ app and services usage compared with Android users? • How do device characteristics affect usage of different genres of apps such as social apps, chat apps, games and media streaming apps? • As larger screens become more common at lower Android smartphone price points, will mobile usage of low and mid tier smartphones rise to match usage of flagship models? © 2014 IHS & Mobidia 2 Key findings • In Q2 2014, 80 percent of smartphone models launched • Smartphone screen sizes have increased dramatically had screens over 4.5 inches, larger than any iPhone in recent years, but until late 2014 Apple lagged its then on sale. And 17% of new smartphones had screen Android competition. sizes of 5.5 inches or greater. • While the iPhone 6 has a screen of 4.7 inches, the • Larger smartphone screen sizes strongly correlate with study shows there is a tipping point for screens around increased minutes of app usage. -

Fnac Reprise
FNAC REPRISE Liste des smartphones éligibles au programme de reprise au 19/08/2016 ACER LIQUID Z4 APPLE IPHONE 5 BLACK 64GB ACER INCORPORATED LIQUID Z530S APPLE IPHONE 5 WHITE 16GB ACER INCORPORATED LIQUID Z630S APPLE IPHONE 5 WHITE 32GB ALBA ALBA 4.5INCH 5MP 4G 8GB APPLE IPHONE 5 WHITE 64GB ALBA DUAL SIM APPLE IPHONE 5C ALCATEL IDOL 3 8GB APPLE IPHONE 5C BLUE 16GB ALCATEL ONE TOUCH 228 APPLE IPHONE 5C BLUE 32GB ALCATEL ONE TOUCH 903 APPLE IPHONE 5C BLUE 8GB ALCATEL ONE TOUCH 903X APPLE IPHONE 5C GREEN 16GB ALCATEL ONE TOUCH IDOL 2 MINI S APPLE IPHONE 5C GREEN 32GB ALCATEL ONE TOUCH TPOP APPLE IPHONE 5C GREEN 8GB ALCATEL ONETOUCH POP C3 APPLE IPHONE 5C PINK 16GB AMAZON FIRE PHONE APPLE IPHONE 5C PINK 32GB APPLE APPLE WATCH EDITION 42MM APPLE IPHONE 5C PINK 8GB APPLE IPHONE 3G APPLE IPHONE 5C WHITE 16GB APPLE IPHONE 3G BLACK 16GB APPLE IPHONE 5C WHITE 32GB APPLE IPHONE 3G BLACK 8GB APPLE IPHONE 5C WHITE 8GB APPLE IPHONE 3G WHITE 16GB APPLE IPHONE 5C YELLOW 16GB APPLE IPHONE 3GS APPLE IPHONE 5C YELLOW 32GB APPLE IPHONE 3GS 8GB APPLE IPHONE 5C YELLOW 8GB APPLE IPHONE 3GS BLACK 16GB APPLE IPHONE 5S APPLE IPHONE 3GS BLACK 32GB APPLE IPHONE 5S BLACK 16GB APPLE IPHONE 3GS WHITE 16GB APPLE IPHONE 5S BLACK 32GB APPLE IPHONE 3GS WHITE 32GB APPLE IPHONE 5S BLACK 64GB APPLE IPHONE 4 APPLE IPHONE 5S GOLD 16GB APPLE IPHONE 4 BLACK 16GB APPLE IPHONE 5S GOLD 32GB APPLE IPHONE 4 BLACK 32GB APPLE IPHONE 5S GOLD 64GB APPLE IPHONE 4 BLACK 8GB APPLE IPHONE 5S WHITE 16GB APPLE IPHONE 4 WHITE 16GB APPLE IPHONE 5S WHITE 32GB APPLE IPHONE 4 WHITE 32GB APPLE IPHONE -

PDF 文件使用"Pdffactory Pro" 试用版本创建
X≤4.0" 4.0"<X≤4.5" 4.5"<X≤5.0" Mobile telephone nokia 3250 nokia E90(双膜) nokia N-GAGE QD nokia 6600F(双膜) nokia Lumia 710 nokia 2100 nokia N9 nokia X7 nokia 6370 nokia N97/N97i nokia 801T nokia 1110 nokia N97(摄像头) nokia Lumia 610 nokia 3310 nokia E7 nokia 3120 nokia N900 samsung i8150 Galaxy W nokia 1100 nokia 603 samsung M920 Transform nokia 6610 nokia N93i(双膜) samsung S5830 Galaxy Ace/I579/I589 nokia 6020/6030 samsung W589(双膜) nokia 3100 samsung S5250/S5253/Wave 525 samsung Wave M S7250/Wave 725/S7250D nokia 6230 samsung B7620U samsung I897 Captivate/I9088/I9010 nokia 6230i samsung S5330/S5333/wave 533/Wavesamsung 2 Pro T959 Vibrant/Galaxy S 4G/i9000 nokia 3220 samsung F708/F700 samsung S5690 Galaxy Xcover nokia 5500 samsung S5750e/S5753/wave 575 samsung I9000 Galaxy S/M110S/I9008/I9001 nokia 5070 samsung S5360/Galaxy Y/I509 samsung S8600 Wave 3/Wave III nokia 2630 samsung F508/F500(双膜) samsung W699(双膜) nokia 6060 samsung i8000 Omnia 2 samsung B7732(双膜) nokia 5030XR samsung i7680 samsung G400(三片) nokia 6151 samsung S8530 Wave II samsung I9020/I9023/Google Nexus S nokia 1112 samsung S5660 Galaxy Gio/I569samsung I8700 Omnia 7 nokia 7360 samsung S5380 Wave Y samsung I909 Galaxy S(跟I9000不同) nokia 1200 samsung R910 Galaxy Indulge 4G/samsung Epic 4G/Galaxy S Pro nokia 1600 samsung i8180c samsung i8350 Omnia W nokia C3-01/C3 Touchsamsung and Type S5820 samsung S7500 Galaxy Ace Plus nokia 6070 samsung R810 Finesse samsung i8160 Galaxy Ace 2 nokia 7250 samsung S5368 samsung i619 Galaxy Ace Dear nokia E50 Samsung S6102 Galaxy Y Duos samsung B9062 -
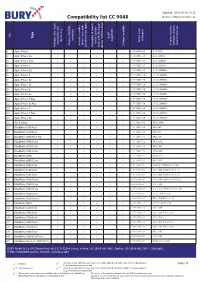
Compatibility List CC 9048 Device Software Version: 22
Updated: 2016-10-25 / V.22 Compatibility list CC 9048 Device software version: 22 No Type A2DP activation (Charger) supported device key Article code car kit / private connection with test/ Comments phone voice-dial software used to Phone‘s REDIAL Phone‘s REDIAL Version of phone Version Access to mobile Charger available Possibility to switch Activation Bluetooth function (voice tags) mode with Bluetooth device / phones keys 1 Apple iPhone 0-07-0258-0.07 3.0(7A341) 2 Apple iPhone 3G 0-07-0258-0.07 4.2.1 (8A306) 3 Apple iPhone 3GS 0-07-0258-0.07 6.1.2 (10B146) 4 Apple iPhone 4 0-07-0258-0.07 7.0.2 (11A501) 5 Apple iPhone 4S 0-07-0258-0.07 7.0.4 (11B554a) 6 Apple iPhone 5 0-07-0258-0.08 10.0.2 (14A456) 7 Apple iPhone 5c 0-07-0258-0.08 10.0.2 (14A456) 8 Apple iPhone 5s 0-07-0258-0.08 10.0.2 (14A456) 9 Apple iPhone 6 0-07-0258-0.08 10.0.2 (14A456) 10 Apple iPhone 6s 0-07-0258-0.08 10.0.2 (14A456) 11 Apple iPhone 6 Plus 0-07-0258-0.08 10.0.2 (14A456) 12 Apple iPhone 6s Plus 0-07-0258-0.08 10.0.2 (14A456) 13 Apple iPhone 7 0-07-0258-0.08 10.0.3 (14A551) 14 Apple iPhone 7 Plus 0-07-0258-0.08 10.0.3 (14A551) 15 Apple iPhone SE 0-07-0258-0.08 10.0.2 (14A456) 16 ASUS Solaris 0-07-0258-0.02 V4.2.2 WWE 17 BlackBerry 8100 Pearl 0-07-0258-0.02 V4.5.0.69 18 BlackBerry 8110 Pearl 0-07-0258-0.02 V4.5.0.55 19 BlackBerry 8220 Pearl Flip 0-07-0258-0.01 V4.6.0.94 20 BlackBerry 8300 Curve 0-07-0258-0.02 V4.5.0.182 21 BlackBerry 8310 Curve