Proceedings of the 23Rd Biennial Pronghorn Workshop 2008
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mountains, Streams, and Lakes of Oklahoma I
Information Series #1, June 1998 Mountains, Streams, and Lakes of OklahomaI Kenneth S. Johnson2 INTRODUCTION valleys, hills, and plains throughout most of the re mainder of Oklahoma (Fig. 1). All the major lakes and Mountains and streams define the landscape of reservoirs of Oklahoma are man-made, and they are Oklahoma (Fig. 1). The mountains consist mainly of important for flood contr()l, water supply, recreation, resistant rock masses that were folded, faulted, and and generation of hydroelectric power. Natural lakes thrust upward in the geologic past (Fig. 2), whereas in Oklahoma are limited to oxbow lakes along major the streams have persisted in eroding less-resistant streams and to playa lakes in the High Plains region rock units and lowering the landscape to form broad of the west. Alphabetical List of20 Lakes with Largest Surface Area (from Oklahoma Water Atlas, Oklahoma Water Resources Board) 1. Broken Bow 11. Lake 0' The Cherokees 2. Canton 12. Oologah 3. Eufaula 13. Robert s. Kerr 4. Fort Gibson 14. Sardis 5. Foss 15. Skiatook 6. Great Salt Plains 16. Tenkiller Ferry ·7. Hudson 17. Texoma 8. Hugo 18. Waurika 9. Kaw 19. Webbers Falls 10. Keystone 20. Wister Modified from Historical Atlas of Oklahoma, by John W. Morris, Charles R. Goins, and Edwin C. 25 McReynolds. Copyright © 1986 by the University I of Oklahoma Press. o 40 80Km Figure 1. Mountains, streams, and principal lakes of Oklahoma. lReprinted from Oklahoma Geology Notes (1993), vol. 53, no. 5, p. 180-188. The Notes article was reprinted and expanded from Oklahoma Almanac, 1993-1994, Oklahoma Department of Lihraries, p. -

Educator's Guide
Educator’s Guide the jill and lewis bernard family Hall of north american mammals inside: • Suggestions to Help You come prepared • essential questions for Student Inquiry • Strategies for teaching in the exhibition • map of the Exhibition • online resources for the Classroom • Correlations to science framework • glossary amnh.org/namammals Essential QUESTIONS Who are — and who were — the North as tundra, winters are cold, long, and dark, the growing season American Mammals? is extremely short, and precipitation is low. In contrast, the abundant precipitation and year-round warmth of tropical All mammals on Earth share a common ancestor and and subtropical forests provide optimal growing conditions represent many millions of years of evolution. Most of those that support the greatest diversity of species worldwide. in this hall arose as distinct species in the relatively recent Florida and Mexico contain some subtropical forest. In the past. Their ancestors reached North America at different boreal forest that covers a huge expanse of the continent’s times. Some entered from the north along the Bering land northern latitudes, winters are dry and severe, summers moist bridge, which was intermittently exposed by low sea levels and short, and temperatures between the two range widely. during the Pleistocene (2,588,000 to 11,700 years ago). Desert and scrublands are dry and generally warm through- These migrants included relatives of New World cats (e.g. out the year, with temperatures that may exceed 100°F and dip sabertooth, jaguar), certain rodents, musk ox, at least two by 30 degrees at night. kinds of elephants (e.g. -

Pbmr-400 Benchmark Solution of Exercise 1 and 2 Using the Moose Based Applications: Mammoth, Pronghorn
EPJ Web of Conferences 247, 06020 (2021) https://doi.org/10.1051/epjconf/202124706020 PHYSOR2020 PBMR-400 BENCHMARK SOLUTION OF EXERCISE 1 AND 2 USING THE MOOSE BASED APPLICATIONS: MAMMOTH, PRONGHORN Paolo Balestra1, Sebastian Schunert1, Robert W Carlsen1, April J Novak2 Mark D DeHart1, Richard C Martineau1 1Idaho National Laboratory 955 MK Simpson Blvd, Idaho Falls, ID 83401, USA 2University of California, Berkeley 2000 Carleton Street, Berkeley, CA 94720, USA [email protected], [email protected], [email protected], [email protected], [email protected], [email protected] ABSTRACT High temperature gas cooled reactors (HTGR) are a candidate for timely Gen-IV reac- tor technology deployment because of high technology readiness and walk-away safety. Among HTGRs, pebble bed reactors (PBRs) have attractive features such as low excess reactivity and online refueling. Pebble bed reactors pose unique challenges to analysts and reactor designers such as continuous burnup distribution depending on pebble mo- tion and recirculation, radiative heat transfer across a variety of gas-filled gaps, and long design basis transients such as pressurized and depressurized loss of forced circulation. Modeling and simulation is essential for both the PBR’s safety case and design process. In order to verify and validate the new generation codes the Nuclear Energy Agency (NEA) Data bank provide a set of benchmarks data together with solutions calculated by the participants using the state of the art codes of that time. An important milestone to test the new PBR simulation codes is the OECD NEA PBMR-400 benchmark which includes thermal hydraulic and neutron kinetic standalone exercises as well as coupled exercises and transients scenarios. -

Cactus (Opuntia Spp.) As Forage 169
Cactus (Opuntia spp.) as forage 169 Food •••A.gricultv,.. Org•nU.taon or United -N••lon• FAO Cactus (Opuntiaspp.) PLANT PRODUCTION as forage AND PROTECTlON PAPER 169 Ed~ed by Candelario Mondragon-Jacobo lnstituto Nacional de Investigaciones Forestales y Agropecuarias (INIFAP) Mexico and Salvador Perez-Gonzalez Universidad Aut6noma de Queretaro Mexico Coordinated for FAD by Enrique Arias Horticultural Crops Group Stephen G. Reynolds Grassland and Pasture Crops Group FAO Plant Production and Protection Division and Manuel D. sanchez Feed Resources Group FAO Animal Production and HeaHh Division Produced within the frameworl< of the FAO International Technical Cooperation Networl< ot on Cactus Pear ••u nttttd• NaUon• Rome,2001 Reprinted 2002 The designations “developed” and “developing” economies are intended for statistical convenience and do not necessarily express a judgement about the stage reached by a particular country, country territory or area in the development process. The views expressed herein are those of the authors and do not necessarily represent those of the Food and Agriculture Organization of the United Nations or of their affiliated organization(s). The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. ISBN 92-5-104705-7 All rights reserved. Reproduction and dissemination of material in this information product for educational or other non-commercial purposes are authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. -

Plant Checklist These Details May Help You Identify It
Urban Landscape Shortgrass Prairie Ecosystem Lowland Riparian Ecosystem The urban landscape is human-built. Interspersed with homes, Minimal rainfall (less than 15 inches a year), ever-present winds The lowland riparian ecosystem is characterized by narrow offices, roads, and other infrastructure are yards, community and a treeless landscape make up the shortgrass prairie. Plants bands of distinctive vegetation along the margins of streams, green spaces, local parks and natural areas. Plant life in the here generally grow low to the ground, are drought tolerant and rivers, ponds, and lakes. It is lower in elevation, and often urban landscape may benefit from additional stormwater have extensive root systems. shaded from sun and wind by abundant trees and shrubs. All runoff and added fertilizers, but it may also be subject to plant life here is governed and shaped by water. frequent disturbance and human manipulation. Because urban Shrubs landscape plants can come from many different places, this list Rabbitbrush (Chrysothamnus nauseosus) Trees addresses common “volunteer” species in unmaintained areas. *Fringed sage (Artemisia frigida) Plains cottonwood (Populus deltoides) Snakeweed (Gutierrezia sarothrae) Narrowleaf cottonwood (Populus angustifolia) Trees Four-winged saltbush (Atriplex canescens) Peach-leaved willow (Salix amygdaloides) Smooth sumac (Rhus glabra) Winterfat (Krascheninnikovia lanata) Yucca (Yucca glauca) **Siberian elm (Ulmus pumila) Shrubs **Russian-olive (Elaeagnus angustifolia) Chokecherry (Padus virginiana) -
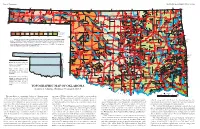
TOPOGRAPHIC MAP of OKLAHOMA Kenneth S
Page 2, Topographic EDUCATIONAL PUBLICATION 9: 2008 Contour lines (in feet) are generalized from U.S. Geological Survey topographic maps (scale, 1:250,000). Principal meridians and base lines (dotted black lines) are references for subdividing land into sections, townships, and ranges. Spot elevations ( feet) are given for select geographic features from detailed topographic maps (scale, 1:24,000). The geographic center of Oklahoma is just north of Oklahoma City. Dimensions of Oklahoma Distances: shown in miles (and kilometers), calculated by Myers and Vosburg (1964). Area: 69,919 square miles (181,090 square kilometers), or 44,748,000 acres (18,109,000 hectares). Geographic Center of Okla- homa: the point, just north of Oklahoma City, where you could “balance” the State, if it were completely flat (see topographic map). TOPOGRAPHIC MAP OF OKLAHOMA Kenneth S. Johnson, Oklahoma Geological Survey This map shows the topographic features of Oklahoma using tain ranges (Wichita, Arbuckle, and Ouachita) occur in southern contour lines, or lines of equal elevation above sea level. The high- Oklahoma, although mountainous and hilly areas exist in other parts est elevation (4,973 ft) in Oklahoma is on Black Mesa, in the north- of the State. The map on page 8 shows the geomorphic provinces The Ouachita (pronounced “Wa-she-tah”) Mountains in south- 2,568 ft, rising about 2,000 ft above the surrounding plains. The west corner of the Panhandle; the lowest elevation (287 ft) is where of Oklahoma and describes many of the geographic features men- eastern Oklahoma and western Arkansas is a curved belt of forested largest mountainous area in the region is the Sans Bois Mountains, Little River flows into Arkansas, near the southeast corner of the tioned below. -

Pronghorn G TAG
ANTELOPE AND ... the American “antelope”! IRAFFE Pronghorn G TAG Why exhibit pronghorns? • Celebrate our local biodiversity by displaying the last surviving species of the Antilocapridae, a mammalian family endemic to North America. • Participate in a recovery program close to home: AZA maintains a priority insurance population of the critically endangered peninsular pronghorn. • Engage guests with these “antelope” from the familiar song “Home on the Range” (even though pronghorns aren’t true “antelope” at all!). • Let visitors get hands-on with the unusual horn sheaths of pronghorns - they are keratinous like horns, but are shed annually like antlers! • Seek partnerships with local running groups: pronghorn are the fastest land animals in North America, able to cover 6 miles in 9 minutes! • TAG Recommendation: Contact the SSP for guidance regarding which pronghorn program is best suited to your facility’s climate. MEASUREMENTS IUCN LEAST Length: 4.5 feet CONCERN Height: 3 feet (CITES I) Stewardship Opportunities at shoulder Peninsular Pronghorn Recovery Project Weight: 65-130 lbs <200 peninsular Contact Melodi Tayles: [email protected] Prairies North America in the wild Care and Husbandry RED SSP (peninsular): 25.26 (51) in 7 AZA institutions (2019) Species coordinator: Melodi Tayles, San Diego Zoo Safari Park [email protected] ; (760) 855-1911 CANDIDATE Program (generic): 34.61 (95) in 21 institutions (2014) Social nature: Herd living. Harem groups with a single male are typical. Bachelor groups may be successful in the absence of females. Mixed species: Pronghorn are frequently exhibited with bison. They have also been housed with camels, deer, cranes, and waterfowl. Housing: Peninsular pronghorn are heat tolerant and do well in windy conditions, but heated shelters recommended where temperatures fall below 40ºF for extended periods. -

GNUSLETTER Volume 37 Number 1
GNUSLETTER Volume 37 Number 1 ANTELOPE SPECIALIST GROUP July 2020 ISSN 2304-0718 IUCN Species Survival Commission Antelope Specialist Group GNUSLETTER is the biannual newsletter of the IUCN Species Survival Commission Antelope Specialist Group (ASG). First published in 1982 by first ASG Chair Richard D. Estes, the intent of GNUSLETTER, then and today, is the dissemination of reports and information regarding antelopes and their conservation. ASG Members are an important network of individuals and experts working across disciplines throughout Africa and Asia. Contributions (original articles, field notes, other material relevant to antelope biology, ecology, and conservation) are welcomed and should be sent to the editor. Today GNUSLETTER is published in English in electronic form and distributed widely to members and non-members, and to the IUCN SSC global conservation network. To be added to the distribution list please contact [email protected]. GNUSLETTER Review Board Editor, Steve Shurter, [email protected] Co-Chair, David Mallon Co-Chair, Philippe Chardonnet ASG Program Office, Tania Gilbert, Phil Riordan GNUSLETTER Editorial Assistant, Stephanie Rutan GNUSLETTER is published and supported by White Oak Conservation The Antelope Specialist Group Program Office is hosted and supported by Marwell Zoo http://www.whiteoakwildlife.org/ https://www.marwell.org.uk The designation of geographical entities in this report does not imply the expression of any opinion on the part of IUCN, the Species Survival Commission, or the Antelope Specialist Group concerning the legal status of any country, territory or area, or concerning the delimitation of any frontiers or boundaries. Views expressed in Gnusletter are those of the individual authors, Cover photo: Peninsular pronghorn male, El Vizcaino Biosphere Reserve (© J. -

Black Tailed Prairie Dog Black-Footed Ferret Ferruginous Hawk Mountain
Black tailed prairie dog Cynomys ludovicianus Prairie rattlesnake Crotalus viridis Live in short and mixed-grass prairies, and prefer ones that have been grazed. Prefer Dine mostly on small mammals, and occasion- grasses in the summer, and switch to plants ally on frogs and lizards. with thick underground roots in the fall and winter. Black-footed ferret Deer mouse Mustela nigripes Peromyscus maniculatus Nocturnal and solitary, over 90% of its diet is Nocturnal, reproduce year-round, eat seeds, prairie dogs. fruits, arthropods. Ferruginous hawk Badger Buteo regalis Taxidea taxus Endemic to interior North America. Catches Nocturnal and carnivorous. Dines on small, small and medium-sized mammals, also birds ground-dwelling mammals. and insects. Mountain plover Western meadowlark Charadrius montanus Sturnella neglecta A shore bird that prefers open, dry areas. Makes a grassy nest, feeds on seeds and Feeds on insects and other small arthropods. insects. Often associates with cattle. Pronghorn Burrowing owl Antilocapra americana Athene cunicularia Swift herbivores, prefer shrubs, wildflowers Feed on large insects and small rodents. Fre- and lichen to grass. quently lives in abandoned prairie dog bur- rows. Puts dung next to its home to attract beetles, which are then eaten. Swift fox Darkling beetle Vulpes velox Eleodes sutralis An omnivore, it feeds on grasses and fruits as Larvae live in soil and eat roots, seeds, and well as carrion and insects. Dens in open sites detritus. with sandy soils. Blue grama grass Painted lady butterfly Bouteloua gracilis Vanessa virginiensis Both cold and drought tolerant, this is the Larvae feed on various wildflowers, especially most productive grass in short grass prairies. -

Magyar Földrajzi Nevek Angol Nyelvre Fordítása
Magyar földrajzi nevek angol nyelvre fordítása Diplomamunka Térképész mesterszak készítette: Horváth Gábor Roland témavezető: Dr. Gercsák Gábor, egyetemi docens Térképtudományi és Geoinformatikai Tanszék Eötvös Loránd Tudományegyetem 2018. EÖTVÖS LORÁND TUDOMÁNYEGYETEM INFORMATIKAI KAR TÉRKÉPTUDOMÁNYI ÉS GEOINFORMATIKAI TANSZÉK DIPLOMAMUNKA-TÉMA BEJELENTŐ Név: Neptun kód: Szak: térképész MSc Témavezető neve: munkahelyének neve és címe: beosztása és iskolai végzettsége: A dolgozat címe: A témavezetést vállalom. .......................................................... (a témavezető aláírása) Kérem a diplomamunka témájának jóváhagyását. Budapest, 20…………………... ........................................................... (a hallgató aláírása) A diplomamunka-témát az Informatikai Kar jóváhagyta. Budapest, 20…………………… …………………………………….. (témát engedélyező tanszék vezetője) Tartalomjegyzék Címlap ..................................................................................................................................................... 1 Témabejelentő ........................................................................................................................................ 2 Tartalomjegyzék ...................................................................................................................................... 1 Bevezetés ................................................................................................................................................. 2 1. fejezet: A jelenlegi helyzet ............................................................................................................. -

Utah Pronghorn Statewide Management Plan
UTAH PRONGHORN STATEWIDE MANAGEMENT PLAN UTAH DIVISION OF WILDLIFE RESOURCES DEPARTMENT OF NATURAL RESOURCES UTAH DIVISION OF WILDLIFE RESOURCES STATEWIDE MANAGEMENT PLAN FOR PRONGHORN I. PURPOSE OF THE PLAN A. General This document is the statewide management plan for pronghorn in Utah. This plan will provide overall direction and guidance to Utah’s pronghorn management activities. Included in the plan is an assessment of current life history and management information, identification of issues and concerns relating to pronghorn management in the state, and the establishment of goals, objectives and strategies for future management. The statewide plan will provide direction for establishment of individual pronghorn unit management plans throughout the state. B. Dates Covered This pronghorn plan will be in effect upon approval of the Wildlife Board (expected date of approval November 30, 2017) and subject to review within 10 years. II. SPECIES ASSESSMENT A. Natural History The pronghorn (Antilocapra americana) is the sole member of the family Antilocapridae and is native only to North America. Fossil records indicate that the present-day form may go back at least a million years (Kimball and Johnson 1978). The name pronghorn is descriptive of the adult male’s large, black-colored horns with anterior prongs that are shed each year in late fall or early winter. Females also have horns, but they are shorter and seldom pronged. Mature pronghorn bucks weigh 45–60 kilograms (100–130 pounds) and adult does weigh 35–45 kilograms (75–100 pounds). Pronghorn are North America’s fastest land mammal and can attain speeds of approximately 72 kilometers (45 miles) per hour (O’Gara 2004a). -

NORTHERN ARIZONA PROVINCE (024) by W.C
NORTHERN ARIZONA PROVINCE (024) By W.C. Butler INTRODUCTION This province covers about 59,000 sq mi, mostly in the southwestern part of the Colorado Plateau. Significant geologic features include the Grand Canyon, Kaibab Arch, Mogollon Highlands transition zone, Monument Uplift, Defiance Uplift, Black Mesa Basin, Holbrook Basin, and southern edges of the Kaiparowits and Blanding Basins. The stratigraphic section shown for northeastern Arizona has demonstrated the highest petroleum potential in Arizona. See Wilson (1962), Butler (1988a), and Dickinson (1989) for synopses of the province's geology and evolution. The lithologically and structurally complex basement of the Colorado Plateau area evolved from northwest-younging Proterozoic terranes sequentially accreted onto the Archean craton. As much as 12,000 ft of Middle and Late Proterozoic strata is preserved in possible rift-aulacogen depositional settings in central Arizona. Thick, unmetamorphosed, organic-rich Late Proterozoic strata deposited in backarc basins or continental lakes of north-central Arizona and south-central Utah have good petroleum potential. The plateau area, as a passive Paleozoic plate margin and buffered Mesozoic retro-arc platform, has been remarkably tectonically stable during Phanerozoic time. The area is characterized by blanket Paleozoic strata, as much as 6,000 ft thick, consisting of mostly shallow marine clastics and carbonates showing numerous disconformities. These strata accumulated during transgressions and regressions from both the northwest and southeast, onlapping and thinning toward the trans-continental arch – a northeast-trending positive area extending from the northeast into central Arizona. Convergence between North and South American tectonic plates, with reactivation of basement blocks, during the late Paleozoic created the plateau's fault-bounded basins and uplifts.