Sonographic Appearance of Steatocystoma: an Analysis of 14
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
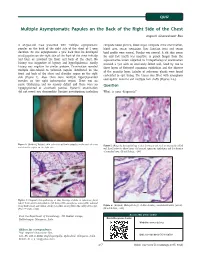
Multiple Asymptomatic Papules on the Back of the Right Side of the Chest Angoori Gnaneshwar Rao
QUIZ Multiple Asymptomatic Papules on the Back of the Right Side of the Chest Angoori Gnaneshwar Rao A 43-year-old male presented with multiple asymptomatic complete blood picture, blood sugar, complete urine examination, papules on the back of the right side of the chest of 1 year blood urea, serum creatinine, liver function tests and serum duration. He was asymptomatic a year back then he developed lipid profile were normal. Fundus was normal. A slit skin smear small papules on the right side of the front of the chest initially for acid fast bacilli was negative. A punch biopsy from the and later on involved the front and back of the chest. No representative lesion subjected to histopathological examination history was suggestive of leprosy and hyperlipidemias. Family revealed a cyst with an intricately folded wall, lined by two to history was negative for similar problem. Examination revealed three layers of flattened squamous epithelium and the absence multiple skin-colored to yellowish papules distributed on the of the granular layer. Lobules of sebaceous glands were found front and back of the chest and shoulder region on the right embedded in cyst lining. The lumen was filled with amorphous side [Figure 1]. Also, there were multiple hyperpigmented eosinophilic material and multiple hair shafts [Figures 2-4]. macules on the right infrascapular region. There was no nerve thickening and no sensory deficit and there were no Question hypopigmented or anesthetic patches. Systemic examination did not reveal any abnormality. Routine investigations including What is your diagnosis? (Original) Multiple skin-colored to yellowish papules on the back of chest Figure 1: Figure 2: (Original) Histopathology of skin showing a cyst with an intricately folded and shoulder region on the right side wall lined by two to three layers of flattened squamous epithelium and the absence of granular layer. -

A Case of Steatocystoma Simplex Involving the Scalp
230 A Case of Steatocystoma Simplex Involving the Scalp Dong Nyeok Hyun, M.D., Jong Hoon Won, M.D., Joon Soo Park, M.D., Hyun Chung, M.D. Department of Dermatology, School of Medicine, Catholic University of Daegu, Daegu, Korea Steatocystoma is a benign adnexal tumor originating from the pilosebaceous duct junction which can be classified into two groups (steatocystoma simplex and steatocystoma multiplex). Steatocystoma simplex, which presents as a solitary lesion, is very rare. Steatocystoma simplex occurs most commonly on the face and the case reported herein involving the scalp is extremely rare. A 49-year-old man presented for evaluation and treatment of a solitary papule on the right parietal scalp which had persisted for a period of 1 year. The histopathologic examination revealed a thin-walled cyst consisting of stratified squamous epithelium with hyaline cuticle that lacked a stratum granulosum. Based on clinical and histologic findings, we diagnosed this case as steatocystoma simplex of the scalp and report this rare case. (Ann Dermatol (Seoul) 20(4) 230∼232, 2008) Key Words: Scalp, Steatocystoma simplex INTRODUCTION CASE REPORT Steatocystoma simplex, first described as a dis- A 49-year-old man presented to our outpatient tinct entity by Brownstein1 in 1982, is an extremely clinic with an asymptomatic papule on the right rare benign adnexal tumor. The individual lesion of parietal scalp which had been present for about 1 steatocystoma simplex is usually identical with that year. The lesion had slowly enlarged a few months of steatocystoma multiplex, both clinically and ago. The physical examination revealed a skin- histologically, but is characterized by solitary, non- colored, deep-seated, soft cystic mass on his right heritable growth in adulthood1. -

The Tamilnadu Dr. M.G.R. Medical University Chennai, Tamil Nadu
CLINICO-PATHOLOGICAL STUDY OF SKIN SURFACE EPIDERMAL AND APPENDAGEAL TUMOURS Dissertation Submitted in partial fulfillment of university regulations for M.D. DEGREE IN DERMATOLOGY, VENEREOLOGY AND LEPROSY BRANCH XII – A THE TAMILNADU DR. M.G.R. MEDICAL UNIVERSITY CHENNAI, TAMIL NADU SEPTEMBER 2006 CERTIFICATE This is to certify that this Dissertation entitled “CLINICO-PATHOLOGICAL STUDY OF SKIN SURFACE EPIDERMAL AND APPENDAGEAL TUMOURS” is a bonafide work done by DR.G.BALAJI, Postgraduate student of Department of Dermatology, Leprosy and Institute of STD, Madras Medical College and Government General Hospital, Chennai – 3 for the award of Degree of M.D.( Dermatology, Venereology and Leprosy ) Branch XII – A during the academic year of 2003-2006. This work has not previously formed in the basis for the award of any degree or diploma. Prof. Dr. B. Parveen, MD., DD., Professor & Head, Dept. of Dermatology and Leprosy, Madras Medical College & Govt. General Hospital, Chennai – 3. Prof. Dr. Kalavathy Ponniraivan, MD., The Dean Madras Medical College & Govt. General Hospital, Chennai – 3. SPECIAL ACKNOWLEDGEMENT I sincerely thank Prof. Dr. Kalavathy Ponniraivan, MD., Dean, Madras Medical College & Govt. General Hospital, Chennai – 3, for granting me permission to use the resources of this institution for my study. ACKNOWLEDGEMENT I sincerely thank Prof. B.Parveen MD.,DD, Professor and Head of Department of Dermatology for her invaluable guidance and encouragement for the successful completion of this study. I express my heart felt gratitude to Dr.N.Gomathy MD.,DD, former Head of department of Dermatology who was instrumental in the initiation of this project, giving constant guidance throughout my work. -

Dermatopathology
Dermatopathology Clay Cockerell • Martin C. Mihm Jr. • Brian J. Hall Cary Chisholm • Chad Jessup • Margaret Merola With contributions from: Jerad M. Gardner • Talley Whang Dermatopathology Clinicopathological Correlations Clay Cockerell Cary Chisholm Department of Dermatology Department of Pathology and Dermatopathology University of Texas Southwestern Medical Center Central Texas Pathology Laboratory Dallas , TX Waco , TX USA USA Martin C. Mihm Jr. Chad Jessup Department of Dermatology Department of Dermatology Brigham and Women’s Hospital Tufts Medical Center Boston , MA Boston , MA USA USA Brian J. Hall Margaret Merola Department of Dermatology Department of Pathology University of Texas Southwestern Medical Center Brigham and Women’s Hospital Dallas , TX Boston , MA USA USA With contributions from: Jerad M. Gardner Talley Whang Department of Pathology and Dermatology Harvard Vanguard Medical Associates University of Arkansas for Medical Sciences Boston, MA Little Rock, AR USA USA ISBN 978-1-4471-5447-1 ISBN 978-1-4471-5448-8 (eBook) DOI 10.1007/978-1-4471-5448-8 Springer London Heidelberg New York Dordrecht Library of Congress Control Number: 2013956345 © Springer-Verlag London 2014 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifi cally the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfi lms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. Exempted from this legal reservation are brief excerpts in connection with reviews or scholarly analysis or material supplied specifi cally for the purpose of being entered and executed on a computer system, for exclusive use by the purchaser of the work. -

Steatocystoma Multiplex-A Rare Genetic Disorder: a Case Report and Review of the Literature Pathology Section
Case Report DOI: 10.7860/JCDR/2012/4691.2698 Steatocystoma Multiplex-A Rare Genetic Disorder: A Case Report and Review of the Literature Pathology Section HEMLATA T. KAMRA, PRADEEP A. GADGIL, AJAY G. OVHAL, RAHUL R. NARKHEDE ABSTRACT is asymptomatic, is a cosmetic threat to the patient . Only a A 17 years old female presented with multiple asymptomatic few cases of the patients with an autosomal dominant mutation, cutaneous cysts all over body, sparing the head and neck who had keratin 17, have been reported. We are reporting here, region. The microscopic examination of the cysts showed the a case of steatocystoma multiplex in a 17 years old female, features of steatocystoma multiplex. This disorder, although it along with its review of literature. Key Words: Steatocystoma, Autosomal dominant, Radiofrequency probe INTRODUCTION On examination, the dermal cysts were found to be round to oval, Steatocystoma multiplex is a rare genetic disorder with an well defined and smooth surfaced, without a punctum and to vary autosomal dominant type of inheritance which usually presents in in diameter from 2-5mm [Table/Fig-1]. The patient gave a history adolescence or is sporadic in nature. Rare cases with an autosomal of similar lesions in her father too. The systemic and the laboratory dominant pattern of inheritance have been published till now [1]. findings were normal. Sonography revealed multiple nodules The disease presents with multiple asymptomatic cysts on the which were oval in shape, which were relatively well marginated axilla, groin, trunk, scrotum and the proximal extremities because and hypoechoic and with a posterior enhancement. -

Skin-Colored Papules on the Chest
WHAT’S YOUR DIAGNOSIS? The timing and location of presentation ABOUT THIS CONDITION can easily be mistaken for acne vulgaris, but Steatocystoma lesions are benign and thought steatocystoma lesions are true sebaceous to arise from a mutation in keratin 17. The Skin-Colored Papules on the Chest cysts, which are rare, and spontaneous res- mutation can be inherited in an autosomal olution with increasing age does not typi- dominant pattern, but sporadic nonheritable LT Aaron S. Cantor, MD; LCDR Michael L. Crandall, MD, FAAD; and LCDR Leah K. Spring, DO, FAAD cally occur. The diagnosis of steatocystoma cases are more common.5 There are no dis- often goes unreported because the disease tinct associations with gender or ethnicity. An otherwise healthy male presents with multiple smooth uniform painless cystic papules is usually asymptomatic and mimics more The dermal cysts arise from the sebaceous scattered across his central chest. common benign skin conditions, so an ac- ducts of the pilosebaceous unit, and histo- curate prevalence and incidence are both pathology typically shows numerous mature Aaron Cantor is a General unknown. sebaceous cells encased by a thin wall of strat- Medical Officer at the 2d 25-year-old man presented with multiple uniform painless cystic pap- First on the differential diagnosis is acne ified squamous epithelium.2 Immunohisto- Marine Logistics Group; and Michael Crandall and multiple sternal cysts that he first ules scattered across his central chest that vulgaris, which also presents at puberty and chemical staining for the defective keratin can Leah Spring are Derma- Anoticed when he was aged 18 years were smooth and flesh-colored to slightly affects nearly 85% of adolescents. -

Atypical Steatocystoma Multiplex with Calcification
International Scholarly Research Network ISRN Dermatology Volume 2011, Article ID 381901, 3 pages doi:10.5402/2011/381901 Case Report Atypical Steatocystoma Multiplex with Calcification Muhammad Hasibur Rahman,1 Muhammad Saiful Islam,2 and Nazma Parvin Ansari3 1 Cosmoderma Skin Cure Center 96/G, Nirmalabas, Sheora Road, Mymensingh 2200, Bangladesh 2 Department of Orthopedics, Community Based Medical College, Mymensingh 2200, Bangladesh 3 Department of Pathology, Community Based Medical College, Mymensingh 2200, Bangladesh Correspondence should be addressed to Muhammad Hasibur Rahman, dr [email protected] Received 17 January 2011; Accepted 7 March 2011 Academic Editors: A. Alomar, M. Feinmesser, C.-C. Lan, and W. Vanscheidt Copyright © 2011 Muhammad Hasibur Rahman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. A 60-year-old male reported to us with an atypical case of giant steatocystoma multiplex in the scrotum with calcification. There was no family history of similar lesions. Yellowish, creamy material was expressed from a nodule during punch biopsy. The diagnosis was based on clinical as well as histological findings. Successful surgical excision was done to cure the case without any complications. 1. Introduction steatocystoma multiplex with extensive calcification and firm adhesion to the scrotum. Steatocystoma multiplex occurs as numerous, epithelial- lined, sebum-filled dermal cysts with characteristic seba- 2. Case Report ceous glands in the cyst walls [1]. Usually it begins in adoles- A 60-year-old male presented to us with asymptomatic cence or early adult life. -

Cutaneous Cysts with Nail Dystrophy in a Young Female: a Classical Association
1/24/2018 Cutaneous Cysts with Nail Dystrophy in a Young Female: A Classical Association Indian J Dermatol. 2017 Nov-Dec; 62(6): 661–664. PMCID: PMC5724318 doi: 10.4103/ijd.IJD_473_16 Cutaneous Cysts with Nail Dystrophy in a Young Female: A Classical Association Romana Ghosh, Kingshuk Chatterjee, Jayanta Kumar Barua, and Anupam Roy From the Department of Dermatology, Venereology and Leprosy, School of Tropical Medicine, Kolkata, West Bengal, India rd Address for correspondence: Dr. Romana Ghosh, 2 No. Debigarh, 3 Sarani, East Jheel, Madhyamgram, Kolkata - 700 129, West Bengal, India. E-mail: [email protected] Received 2016 Aug; Accepted 2017 Aug. Copyright : © 2017 Indian Journal of Dermatology This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms. Abstract Pachyonychia Congenita (PC) refers to a group of autosomal dominant disorders with variable clinical presentations. While nail dystrophy and plantar keratoderma are the most consistent features in all the variants, a myriad of other manifestations has been observed. This report highlights a case of young female presenting with multiple asymptomatic cutaneous cysts associated with plantar kearatoderma and nail dystrophy. Similar nail changes were evident in her son also. Such clinical presentation, in corroboration with histopathological evaluation of the cutaneous cyst prompted us to make a diagnosis of Pachyonychia Congenita type II. Keywords: Autosomal dominant, pachyonychia congenita, plantar keratoderma, steatocystoma multiplex What was known? Pachyonychia Congenita is a rare, autosomal dominant genodermatosis characterized by dystrophy of the nails associated with nail bed and hyponychial hyperkeratosis, of which type II has been associated with muta tio ns in K17. -

Aars Hot Topics Member Newsletter
AARS HOT TOPICS MEMBER NEWSLETTER American Acne and Rosacea Society 201 Claremont Avenue • Montclair, NJ 07042 (888) 744-DERM (3376) • [email protected] www.acneandrosacea.org Like Our YouTube Page Visit acneandrosacea.org to Become an AARS Member and TABLE OF CONTENTS Donate Now on Industry News acneandrosacea.org/donate Galderma and Aklief unveil "Me Being Me" campaign ............................................... 2 Ortho Dermatologics opens applications for 2021 Aspire Higher ............................... 2 Our Officers New Medical Research Hidradenitis suppurativa in the pediatric population ................................................... 3 J. Mark Jackson, MD Clinical evaluation of the efficacy of a facial serum .................................................... 4 AARS President Combination of 5-Aminolevulinic acid photodynamic therapy and isotretinoin ........... 4 Zinc(II) complexes of amino acids as new active ingredients ..................................... 5 Andrea Zaenglein, MD Vulvar hidradenitis suppurativa ................................................................................... 5 AARS President-Elect Oral clindamycin and rifampicin in the treatment of hidradenitis suppurativa ............ 5 A comparative study between once-weekly and alternating twice-weekly regimen ... 6 Joshua Zeichner, MD Clascoterone: A novel topical androgen receptor inhibitor for the treatment of acne . 6 AARS Treasurer Epithelialized tunnels are a source of inflammation in hidradenitis suppurativa......... 7 Bethanee Schlosser, -

CASE REPORTS Steatocystoma Multiplex of Scortum- Rare Genetic Disorder: a Case Report and Review of Literature MM Sahaa, S Sahab, RLD Banikc, MM Hossaind
CASE REPORTS Steatocystoma Multiplex of Scortum- Rare Genetic Disorder: A Case Report and Review of Literature MM SAHAa, S SAHAb, RLD BANIKc, MM HOSSAINd Summary: cosmetic threat to the patient. Only a few cases of the patients A 25 years old male attended the skin & VD outpatient with an autosomal dominant mutation, who had keratin department of Khulna Medical College Hospital on 16th 17; have been reported. We are reporting here a case of June, 2013 with complaints of multiple asymptomatic small steatocystoma multiplex of scortum in a 25 years old male rounded firm, cystic nodules that are adherent to the along with review of literature. overlying skin of scortum. The microscopic examination Key words: Steatocystoma multiplex, Genetic disorder. of the cystic nodules showed the features of steatocystoma (J Banagladesh Coll Phys Surg 2015; 33: 218-221) multiplex. This disorder, although it is asymptomatic, is a Introduction: referred to as steatocystoma simplex7. Steatocystoma Steatocystoma multiplex was first described by Jamieson multiplex present with early dome shaped lesion that in 1873, and the term was coined by pringle in 18991. are translucent and which change to a yellowish colour Steatocystoma multiplex is very uncommon genetic with age. The puncta are not obvious but the comedeones are an associated featrue8. The disorder which usually begins in adolescence and early spontaneous rupture of the cyst, if it occurs, with result 2 adult life . The condition is inherited as an autosomal in steatocystoma multiplex suppurativum which is 3 dominant in many cases . Both sexes are affected characterised by inflammation and scarring which is equally4. -

A Clinical Study of Non Venereal Genital Dermatoses
A CLINICAL STUDY OF NON VENEREAL GENITAL DERMATOSES Dissertation submitted to THE TAMILNADU DR. M.G.R. MEDICAL UNIVERSITY CHENNAI – 600 032 APRIL - 2015. In partial fulfillment of the regulations required for the award of M.D. DEGREE IN DERMATOLOGY, VENEREOLOGY AND LEPROLOGY BRANCH XII DEPARTMENT OF DERMATOLOGY Coimbatore Medical College Hospital, Coimbatore. DECLARATION I Dr.A.N.M.Maalik babu solemnly declare that the dissertation entitled “A clinical study of non venereal genital dermatoses” was done by me in the Department of Dermatology and Venereology at Coimbatore Medical College Hospital during the period from August 2013 to July 2014 under the guidance & supervision of Dr.P.P.Ramasamy M.D.,D.D., Professor & Head of Department, Department of Dermatology and Venereology ,Coimbatore Medical College Hospital ,Coimbatore. The dissertation is submitted to Tamil nadu Dr.MGR Medical University,Chennai towards the partial fulfillment of the requirement for the award of M.D., degree in Dermatology, Venereology and Leprology. I have not submitted this dissertion on any previous occasion to any university for the award of any degree. PLACE: Dr. A.N.M.Maalik babu DATE: CERTIFICATE This is to certify that the dissertation entitled “A clinical study of non venereal genital dermatoses ” is a record of bonafide work done by Dr.A.N.M.Maalik babu , Post graduate student in the Department of Dermatology , Venereology and Leprology , Coimbatore Medical College Hospital,Coimbatore under the guidance of Dr.P.P.Ramasamy M.D.,D.D., Professor & Head of Department, Department of Dermatology, Coimbatore Medical College Hospital ,Coimbatore in partial fulfillment of the regulations of the Tamilnadu DR.M.G.R Medical University, Chennai towards the award of M.D., degree (Branch XII) in Dermatology, Venereology and Leprology. -

Steatocystoma Multiplex: a Case Report of a Rare Entity
Imaging Science in Dentistry 2019; 49: 317-21 https://doi.org/10.5624/isd.2019.49.4.317 Steatocystoma multiplex: A case report of a rare entity Nan-Young Shin 1, Ju Hee Kang 2, Jo-Eun Kim 2, Khantaly Symkhampa 1,3, Kyung-Hoe Huh 1, Won-Jin Yi 1, Min-Suk Heo 1,*, Sam-Sun Lee 1, Soon-Chul Choi 1 1Department of Oral and Maxillofacial Radiology and Dental Research Institute, School of Dentistry, Seoul National University, Seoul, Korea 2Department of Oral and Maxillofacial Radiology, Seoul National University Dental Hospital, Seoul, Korea 3Division of Oral and Maxillofacial Radiology, Department of Basic Science, Faculty of Dentistry, University of Health Sciences, Vientiane, Laos ABSTRACT Steatocystoma multiplex is an uncommon benign skin disease, which typically manifests as numerous intradermal cysts that can be scattered anywhere on the body. Although usually asymptomatic, it can be significantly disfiguring. One type of steatocystoma multiplex is known to be associated with the autosomal dominant inheritance of a mutation in the gene coding for keratin 17 (KRT17). In such cases, it is often concurrent with other developmental abnormalities of the ectoderm-derived tissues, such as the nails, hair, and teeth. To the best of our knowledge, few cases have been reported of steatocystoma multiplex of the oral and maxillofacial region. This report describes a case of steatocystoma multiplex of both sides of the neck and multiple dental anomalies, with a focus on its clinical, radiological, and histopathological characteristics, as well as the possibility that the patient exhibited the familial type of this condition. (Imaging Sci Dent 2019; 49: 317-21) KEY WORDS: Steatocystoma Multiplex; Computed Tomography; Keratin-17 Steatocystoma multiplex is a rare benign subcutaneous Steatocystoma multiplex was first mentioned in a case disease characterized by multiple dermal cyst-like lesions report published by Jamieson in 1873 involving numerous derived from the pilosebaceous glands.