Current Risk for Transfusion Transmitted Infections Roger Y
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
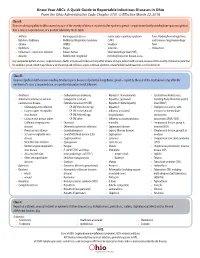
The Know Your Abcs: a Quick Guide to Reportable Infectious Diseases
Know Your ABCs: A Quick Guide to Reportable Infectious Diseases in Ohio From the Ohio Administrative Code Chapter 3701-3; Effective March 22, 2018 Class A: Diseases of major public health concern because of the severity of disease or potential for epidemic spread – report immediately via telephone upon recognition that a case, a suspected case, or a positive laboratory result exists. • Anthrax • Meningococcal disease • Severe acute respiratory syndrome fever, Marburg hemorrhagic fever, • Botulism, foodborne • Middle East Respiratory Syndrome (SARS) and Crimean-Congo hemorrhagic • Cholera (MERS) • Smallpox fever • Diphtheria • Plague • Tularemia • Yellow fever • Influenza A – novel virus infection • Rabies, human • Viral hemorrhagic fever (VHF), • Measles • Rubella (not congenital) including Ebola virus disease, Lassa Any unexpected pattern of cases, suspected cases, deaths or increased incidence of any other disease of major public health concern, because of the severity of disease or potential for epidemic spread, which may indicate a newly recognized infectious agent, outbreak, epidemic, related public health hazard or act of bioterrorism. Class B: Disease of public health concern needing timely response because of potential for epidemic spread – report by the end of the next business day after the existence of a case, a suspected case, or a positive laboratory result is known. • Amebiasis • Carbapenemase-producing • Hepatitis C (non-perinatal) • Spotted Fever Rickettsiosis, • Arboviral neuroinvasive and non- carbapenem-resistant • Hepatitis C (perinatal) including Rocky Mountain spotted neuroinvasive disease: Enterobacteriaceae (CP-CRE) • Hepatitis D (delta hepatitis) fever (RMSF) • Chikungunya virus infection • CP-CRE Enterobacter spp. • Hepatitis E • Staphylococcus aureus, with • Eastern equine encephalitis • CP-CRE Escherichia coli • Influenza-associated resistance or intermediate virus disease • CP-CRE Klebsiella spp. -

Reportable Diseases and Conditions
KINGS COUNTY DEPARTMENT of PUBLIC HEALTH 330 CAMPUS DRIVE, HANFORD, CA 93230 REPORTABLE DISEASES AND CONDITIONS Title 17, California Code of Regulations, §2500, requires that known or suspected cases of any of the diseases or conditions listed below are to be reported to the local health jurisdiction within the specified time frame: REPORT IMMEDIATELY BY PHONE During Business Hours: (559) 852-2579 After Hours: (559) 852-2720 for Immediate Reportable Disease and Conditions Anthrax Escherichia coli: Shiga Toxin producing (STEC), Rabies (Specify Human or Animal) Botulism (Specify Infant, Foodborne, Wound, Other) including E. coli O157:H7 Scrombroid Fish Poisoning Brucellosis, Human Flavivirus Infection of Undetermined Species Shiga Toxin (Detected in Feces) Cholera Foodborne Disease (2 or More Cases) Smallpox (Variola) Ciguatera Fish Poisoning Hemolytic Uremic Syndrome Tularemia, human Dengue Virus Infection Influenza, Novel Strains, Human Viral Hemorrhagic Fever (Crimean-Congo, Ebola, Diphtheria Measles (Rubeola) Lassa, and Marburg Viruses) Domonic Acid Poisoning (Amnesic Shellfish Meningococcal Infections Yellow Fever Poisoning) Novel Virus Infection with Pandemic Potential Zika Virus Infection Paralytic Shellfish Poisoning Plague (Specify Human or Animal) Immediately report the occurrence of any unusual disease OR outbreaks of any disease. REPORT BY PHONE, FAX, MAIL WITHIN ONE (1) WORKING DAY Phone: (559) 852-2579 Fax: (559) 589-0482 Mail: 330 Campus Drive, Hanford 93230 Conditions may also be reported electronically via the California -

STI Screening Timetable
Patient Education Information from University Health Center’s STI Screening Clinic Page 1 of 1 STI Screening Timetable How long until STI (sexually transmitted infection) screening tests turn positive? How long until STI symptoms might show up? The time between infection and a positive test, or between infection and symptoms, is variable and depends on many factors, including the behavior of the infectious agent, how and where the body is infected, and the state of a person’s immune system and personal health. Many STIs don’t have any symptoms. The incubation period times listed in the chart below are averages only. If you have further questions or concerns, you can schedule an appointment with a clinician at 541-346-2770. STI screening test Window period (time from exposure until Incubation period (time between exposure and screening test turns positive) when symptoms appear) Chlamydia (urine specimen or swab of 1 week most of the time Often no symptoms vagina, rectum, throat) 2 weeks catches almost all 1-3 weeks on average Gonorrhea (urine specimen on swab of 1 week most of the time Often no symptoms, especially vaginal vagina, rectum, throat) 2 weeks catches almost all infections usually within 2-8 days but can be up to 2 weeks Syphilis (blood test, RPR) 1 month catches most Often symptoms too mild to notice 3 months catches almost all 10-90 days average 21 days HIV (oral cheek swab) 1 month catches most Sometimes mild body aches and fever within 1-2 3 months catches almost all weeks then can be months to years HIV (blood test, antigen/antibody -

Hepatitis B and C Testing: Why? Who? How? a Guidance Paper on Testing in Community and Harm Reduction Settings 1 Colophon
Hepatitis B and C testing: why? who? how? A guidance paper on testing in community and harm reduction settings 1 Colophon This paper is a product of the Correlation Hepatitis C Initiative. You can access the paper at www.hepatitis-c-initiative.eu Author: Danny Morris Review: M. Harris, A. Kautz, A. Leicht, H. Lochtenberg, E. Schatz Copyright © 2016 Copyrights remains with the publisher Correlation Network PO Box 10887 1001 EW Amsterdam The Netherlands Phone.: +31 20 5317600 Fax.: +31 20 4203528 [email protected] Correlation Network is a part of the international activities of the Regenboog Groep. For more information: www.deregenboog.org The production of this paper has been supported by an unrestricted grant from Gilead Sciences Europe Ltd 2 Acknowledgements We want to thank the author Danny Morris and all who helped to draft this paper and the Regenboog Group, Abbvie and GILEAD for their financial support of the Hepatitis C Initiative. Eberhard Schatz Correlation Hepatitis C Initiative Amsterdam, December 2016 “The Hepatitis C Initiative aims to enhance the momentum of current HCV treatment opportunities and strives for universal access to essential HCV prevention and treatment for the most affected and under-served communities: people who use drugs.” 3 Content Chapter 1: Introduction 6 1.1 Hepatitis B and C infect one in fifty adults in the European Region 9 1.2 Higher rates of hepatitis among vulnerable groups 9 1.3 A public health approach to hepatitis 9 1.4 Harm reduction as prevention 10 1.5 Diagnosing hepatitis B and C 10 1.6 Awareness raising - key recommendations 11 1.7 Testing and diagnosis - key recommendations 13 Chapter 2: Who should be tested 14 2.1 Barriers to testing 16 2.2 Barriers to testing and treatment may include 16 2.3 Overcoming barriers to testing 17 2.4 Testing as standard practice 18 2.5 Pre and post-test discussion 18 2.6 Serological testing for viral hepatitis 20 4 Chapter 3: Screening technologies and development of non-invasive techniques 22 3.1 Venapuncture 23 3.2 Dried blood spot testing 24 3.3. -

Donor Testing and Risk: Current Prevalence, Incidence, and Residual Risk of Transfusion-Transmissible Agents in US Allogeneic Donations
Donor Testing and Risk: Current Prevalence, Incidence, and Residual Risk of Transfusion-Transmissible Agents in US Allogeneic Donations Shimian Zou, Susan L. Stramer, and Roger Y. Dodd Over the past 20 years, there has been a major increase virus (HIV) and human T-cell lymphotropic virus did in the safety of the blood supply, as demonstrated by not. The incidence (/100 000 person-years) of HIV and declining rates of posttransfusion infection and reduc- HCV among repeat donors showed apparent increases tions in estimated residual risk for such infections. from 1.55 and 1.89 in 2000 through 2001 to 2.16 Reliable estimates of residual risk have been possible and 2.98 in 2007 through 2008. These observed within the American Red Cross system because of the fluctuations confirm the need for continuous monitor- availability of a large amount of reliable and consistent ing and evaluation. The residual risk of HIV, HCV, and data on donations and infectious disease testing human T-cell lymphotropic virus among all allogeneic results. Among allogeneic blood donations, the pre- donations is currently below 1 per 1 million donations, valence rates of infection markers for hepatitis C virus and that of hepatitis B surface antigen is close to 1 per (HCV) and hepatitis B virus have decreased over time, 300 000 donations. although rates for markers of human immunodeficiency © 2012 Elsevier Inc. All rights reserved. HE BLOOD SUPPLY in the United States DEMOGRAPHY OF DONOR POPULATIONS T and many other countries has become safer In 2008, a total of 3 830 094 volunteer blood than ever, thanks to continuing improvements in donors successfully made 6 638 877 blood dona- donor selection and testing, along with broad tions to ARC Blood Services, with whole blood and improvements in public health. -

Sexually Transmitted Infection Prevention and Treatment
Sexually Transmitted Infection Prevention and Treatment Kevin L. Ard, MD, MPH Massachusetts General Hospital, The Fenway Institute Disclosures I have no financial disclosures. Doxycycline PEP is not FDA-approved. Learning objectives 1. Describe the epidemiology of syphilis, chlamydia, gonorrhea, and other STIs among LGBTQ populations 2. Summarize optimal screening strategies for STIs. 3. Outline approaches to STI control that can be integrated into primary care. 3 Caveats ▪ Many (most?) LGBTQ people do not face a high risk of STIs. ▪ Clinical care must be individualized, not based on group risk. ▪ Data about STIs among cisgender WSW are limited. ▪ Terms that describe identity and behavior are imperfect and change over time. The rate of chlamydia diagnosis is increasing. Sexually Transmitted Disease Surveillance 2017, CDC Proportion of STI clinic patients testing positive for chlamydia Sexually Transmitted Disease Surveillance 2017, CDC The rate of gonorrhea diagnosis is increasing. Sexually Transmitted Disease Surveillance 2017, CDC MSM face an increasing disparity in the rate of gonorrhea. Sexually Transmitted Disease Surveillance 2017, CDC Antimicrobial resistance in gonorrhea is increasing. Resistanc e Elevated MICs Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2017. Atlanta: U.S. Department of Health and Human Services; 2018. Neisseria gonorrhoeae — Percentage of Urethral Isolates with Elevated Azithromycin Minimum Inhibitory Concentrations (MICs) (≥2.0 µg/ml) and Elevated Ceftriaxone MICs (≥0.125 μg/ml) by Reported Sex of Sex Partners, Gonococcal Isolate Surveillance Project (GISP), 2011–2017 A. Azithromycin B. Ceftriaxone * No cases of elevated ceftriaxone MICs were reported among MSM in 2017. ACRONYMS: MSM = Gay, bisexual, and other men who have sex with men (collectively referred to as MSM); MSW = Men who have sex with women only. -

Know Your Abcs: a Quick Guide to Reportable Infectious Diseases in Ohio
Know Your ABCs: A Quick Guide to Reportable Infectious Diseases in Ohio From the Ohio Administrative Code Chapter 3701-3; Effective August 1, 2019 Class A: Diseases of major public health concern because of the severity of disease or potential for epidemic spread – report immediately via telephone upon recognition that a case, a suspected case, or a positive laboratory result exists. • Anthrax • Measles • Rubella (not congenital) • Viral hemorrhagic fever • Botulism, foodborne • Meningococcal disease • Severe acute respiratory (VHF), including Ebola virus • Cholera • Middle East Respiratory syndrome (SARS) disease, Lassa fever, Marburg • Diphtheria Syndrome (MERS) • Smallpox hemorrhagic fever, and • Influenza A – novel virus • Plague • Tularemia Crimean-Congo hemorrhagic infection • Rabies, human fever Any unexpected pattern of cases, suspected cases, deaths or increased incidence of any other disease of major public health concern, because of the severity of disease or potential for epidemic spread, which may indicate a newly recognized infectious agent, outbreak, epidemic, related public health hazard or act of bioterrorism. Class B: Disease of public health concern needing timely response because of potential for epidemic spread – report by the end of the next business day after the existence of a case, a suspected case, or a positive laboratory result is known. • Amebiasis • Carbapenemase-producing • Hepatitis B (perinatal) • Salmonellosis • Arboviral neuroinvasive and carbapenem-resistant • Hepatitis C (non-perinatal) • Shigellosis -

Hepatitis E Virus Genotype 3 in Shellfish, United Kingdom
LETTERS term to dramatically reduce the high 3. Biswas PK, Christensen JP, Ahmed SS, biologically relevant sentinels and incidence of HPAI in Bangladesh. We Barua H, Das A, Rahman MH. at al. Avian can indicate potential pathogens that infl uenza outbreaks in chickens, Bangla- have progressively and dramatically desh. Emerg Infect Dis. 2008;14:1909–12. are contaminating the environment. increased the scope and benefi ts of our http://dx.doi.org/10.3201/eid1412.071567 It is essential to ensure that this pilot PVC implementation program, 4. Biswas PK, Christensen JP, Ahmed SS, sustainable resource of coastal areas, but additional work is needed. To Das A, Rahman MH, Barua H, et al. where mussels and oysters are farmed Risk for infection with highly pathogenic help spread PVC approaches through- avian infl uenza virus (H5N1) in back- or collected wild, is not subjected to out the country, community leaders, yard chickens, Bangladesh. Emerg In- environmental contamination that imams of local mosques, and school fect Dis. 2009;15:1931–6. http://dx.doi. could lead to public health risks. teachers can be trained to implement org/10.3201/eid1512.090643 Risk management for bivalve 5. Biswas PK, Rahman MH, Das A, Ahmed awareness programs on safe practices SS, Giasuddin M, Christensen JP. Risk mollusks, aimed at control of fecal for raising poultry and regular clean- for highly pathogenic avian infl uenza pollution, relies heavily on the use ing and disinfection of live bird mar- H5N1 virus infection in chickens in of Escherichia coli as an indicator kets. The strengthening of biosecurity small-scale commercial farms, in a high- of fecal (sewage) contamination risk area, Bangladesh, 2008. -

Emerging Water-Borne Pathogens
Appl Microbiol Biotechnol (2003) 61:424–428 DOI 10.1007/s00253-003-1302-y MINI-REVIEW S. Sharma · P. Sachdeva · J. S. Virdi Emerging water-borne pathogens Received: 1 October 2002 / Revised: 27 February 2003 / Accepted: 28 February 2003 / Published online: 9 April 2003 Springer-Verlag 2003 Abstract Emerging water-borne pathogens constitute a number of reviews and other publications available on the major health hazard in both developed and developing subject discuss emerging and re-emerging pathogens in nations. A new dimension to the global epidemiology of general, including water-borne, without addressing the cholera—an ancient scourge—was provided by the issues specifically pertinent to emerging water-borne emergence of Vibrio cholerae O139. Also, water-borne pathogens. This review critically examines which organ- enterohaemorrhagic Escherichia coli (E. coli O157:H7), isms really qualify as emerging water-borne pathogens, although regarded as a problem of the industrialized west, the possible reasons underlying their emergence and has recently caused outbreaks in Africa. Outbreaks of specific measures to face the challenge posed by them. chlorine-resistant Cryptosporidium have motivated water Due to the paucity of data, it may be difficult to decide authorities to reassess the adequacy of current water- which of all water-borne pathogens are emerging. Nev- quality regulations. Of late, a host of other organisms, ertheless, there are some clear-cut candidates. such as hepatitis viruses (including hepatitis E virus), No organism other than Vibrio cholerae could serve as Campylobacter jejuni, microsporidia, cyclospora, Yersin- a better example of an emerging water-borne pathogen. ia enterocolitica, calciviruses and environmental bacteria Cholera is an ancient scourge and to date seven like Mycobacterium spp, aeromonads, Legionella pneu- pandemics have been recorded. -

Disease Reporting-Clinician
Infections, diseases and conditions reportable by clinicians: 2015 Report immediately • Anthrax (Bacillus anthracis) • Botulism (Clostridium botulinum) • Cholera (Vibrio cholerae O1, O139, or toxigenic) • Diphtheria (Corynebacterium diphtheriae) • Hemorrhagic fever caused by viruses of the filovirus (e.g., Ebola, Marburg) or arenavirus (e.g., Lassa, Machupo) families • Influenza (novel)1 (From footnote: Influenza A virus that cannot be subtyped by commercially distributed assays) • Marine intoxication (intoxication caused by marine microorganisms or their byproducts (e.g., paralytic shellfish poisoning, domoic acid intoxication, ciguatera, scombroid) • Measles (rubeola) • Plague (Yersinia pestis) • Poliomyelitis • Rabies (human) • Rubella • SARS (Severe Acute Respiratory Syndrome or SARS-coronavirus) • Smallpox (variola) • Tularemia (Francisella tularensis) • Yellow fever • Outbreaks and uncommon illnesses (any known or suspected common-source outbreak; any uncommon illness of potential public health significance) Report within 24 hours (including weekends and holidays) • Haemophilus influenzae (any isolation or identification from a normally sterile specimen type) • Neisseria meningitidis • Pesticide poisoning Public Health Division | Infections, diseases and conditions reportable by clinicians 128 Report within one working day • Amebic infections (central nervous system only) (for example infection by Naegleria or Balamuthia spp. • Animal bites (of humans) • Arthropod vector-borne disease (babesiosis, California encephalitis, Colorado -

Infant Botulism ESCMID Online Lecture Library
Emerging issues in food-borne infection ‘We never look for it because we never find it’ – emerging pathogens we need to know about ©John by Threlfall author ESCMID Online UKLecture Library ECCMID April 2013 ‘We never look for it because we never find it’ – emerging pathogens we need to know about ‘There are known knowns; there are things we know we know. We also know there are known unknowns; that is to say, we know there are some things we do not know’. ‘But there are also unknown unknowns – the ones we don’t know we don’t know’.© by author ESCMID Online Lecture Library Donald Rumsfeld, US Defence Secretary, 2002 ‘We never look for it because we never find it’ - emerging pathogens we need to know about Questions (1) 1. What is the trigger for recognising the emergence of a ‘new’ pathogen? Possible answers: Increase in incidence above ‘expected’ norm. Outbreak of ‘new’ strain. Increase in mortality in vulnerable population groups. 2. Which ‘emerging pathogen’© by hasauthor been responsible for major outbreak(s) of food-poisoning in Europe over the last five years, involving numerous fatalities? ESCMID Online Lecture Library Possible answers: Norovirus Cryptosporidium Hepatitis E (HEV) Escherichia coli O104:H4 ‘We never look for it because we never find it’ – emerging pathogens we need to know about Disease / Organism • What are the disease symptoms? • What is the causative organism? • How rapidly is this isolated? • How common is the organism? – individuals, communities, environment Source / Reservoir © by author • Is it a food contamination issue? • ESCMIDIf so, what is the food? Online Lecture Library • What is the food animal reservoir (if any?) ‘We never look for it because we never find it’ - emerging pathogens we need to know about Questions (2) 1. -

Using Knowledge of HIV Status As an HIV Prevention Strategy
From Prevention in Focus, Spring 2014 Unknown, negative or positive? Using knowledge of HIV status as an HIV prevention strategy By James Wilton and Tim Rogers As front-line workers in HIV prevention, it is important to understand what prevention strategies clients are using and how they understand the risks associated with them. A common HIV prevention strategy used by gay men and other men who have sex with men (MSM) is known as “serosorting” and involves limiting all – or just “high-risk” – sexual activities to partners who have the same HIV status. For example, an HIV-negative person may choose to only have condomless sex with other people who are HIV negative or an HIV-positive person may choose to only have condomless sex with other people who are HIV positive. This strategy is used in the context of different types of relationships, such as stable, casual, monogamous and non-monogamous relationships. This article focuses on how often serosorting is used, how well it works, and how knowledge of HIV status can be effectively used as a prevention strategy. The article only covers serosorting strategies that are based on individuals identifying themselves or others as HIV-positive or HIV-negative. It does not address other factors, such as viral load or use of other HIV prevention strategies, which play a very important role in assessing HIV risk. For information on these other strategies, please see the resource list at the end of the article. How common is serosorting? Serosorting is quite common among MSM in Canada and other parts of the world.