Experimental Investigation Into the Occurrence and Effects of Individual
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diversity and Conservation of Amphibians and Reptiles in North Punjab, Pakistan
Diversity and conservation of amphibians and reptiles in North Punjab, Pakistan. MUHAMMAD RAIS, SARA BALOCH, JAVERIA REHMAN, MAQSOOD ANWAR, IFTIKHAR HUSSAIN AND TARIQ MAHMOOD Department of Wildlife Management, PMAS Arid Agriculture University Rawalpindi, Pakistan. Corresponding Author: Muhammad Rais, Visiting Scholar, Department of Biology, Indiana-Purdue University Fort Wayne, Indiana, USA. Email: [email protected] ABSTRACT - Amphibians and reptiles are the most neglected and least studied wildlife groups in Pakistan. The present study was conducted in the selected areas of districts Rawalpindi, Islamabad and Chakwal, North Punjab, Pakistan, to obtain data on herpetofaunal species richness and abundance from February, 2010 to January, 2011 using area-constrained searches. A total of 35 species of amphibians and reptiles (29 genera, 16 families, four orders) were recorded from the study area. Of the recorded species, 30 were reptiles (25 genera, 13 families, three orders) and five were amphibians (four genera, three families and a single order). A total of 388 individuals belonging to 11 recognizable taxonomic units (RTUs) with a population density of 0.22 individuals/ ha. and 4.10 encounters were recorded. Of the recorded RTUs, two (lacertids and skinks) were rated as uncommon, seven (hard-shell turtles, soft-shell turtles, agamids, gekkonids, medium and large-sized lizards, non-venomous snakes and venomous snakes) as frequent and two (toads and frogs) as common. Districts Rawalpindi/ Islamabad had higher species richness while District Chakwal had relatively higher species diversity and evenness. Threatened species of the area included the Narrow-headed Soft-shell Turtle (Chitra indica), Indian Soft-shell Turtle (Nilssonia gangetica), Peacock Soft-shell Turtle (Aspideretes hurum), and Brown River Turtle (Pangshura smithii). -

The Indus Equation 2 Introduction CHAPTER 1 Overview of Pakistan’S Water Resources
C-306, Montana, Lokhandwala Complex Andheri West, Mumbai 400 053, India Email: [email protected] www.strategicforesight.com Project Advice Ilmas Futehally Authors Gitanjali Bakshi Sahiba Trivedi Creative Preeti Rathi Motwani Copyright © Strategic Foresight Group, 2011 Permission is hereby granted to quote or reproduce from this report with due credit to Strategic Foresight Group Processed by MadderRed, Mumbai FOREWORD Strategic Foresight Group has been a consistent advocate of reason in relations between India and Pakistan. It has recognised water as a critical determinant of peace and development in many parts of the world. This paper brings together these two strands in our work. It will be in order to recall some of the earlier work done by Strategic Foresight Group to urge rationality in India- Pakistan relations. In 2004, we published the first ever comprehensive assessment of Cost of Conflict between India-Pakistan in a report with this title. In 2005 we published The Final Settlement where we strongly argued that integrated water development would need to be a part of long-lasting solution between the two countries. Since then we have been regularly advocating a pragmatic approach for India and Pakistan to foster cooperation and move ahead to enable social and economic development of their people, instead of wasting precious resources on terrorism, counter terrorism and an arms race. We initiated work on water on the advice of an international conference onResponsibility to the Future, which was co-hosted by SFG with the United Nations Global Compact, inaugurated by the President of India and attended by delegates from 25 countries in June 2008. -

Brief on Water Pollution Water – the Essential Resource
Brief on Water Pollution Water – the essential resource Fresh water as a commodity generates concern being an exhaustible resource and due to the environmental issues related to its degradation. Preserving the quality and availability of freshwater resources however, is becoming the most pressing of many environmental challenges for Pakistan. Perhaps, because water is considered a cheap readily available resource, there is not enough appreciation just how much stress human demands for water are placing on natural ecosystems. Pressures The stress on water resources is from multiple sources and the impact can take diverse forms. The growth of urban megalopolises, increased industrial activity and dependence of the agricultural sector on chemicals and fertilizers has led to the overcharging of the carrying capacity of our water bodies to assimilate and decompose wastes. Deterioration in water quality and contamination of lakes, rivers and ground water aquifers has therefore resulted. The following sub-sections present a closer analysis of various sources of pressure on the country’s water resource base. Increasing Population In 2004, Pakistan stated a population growth rate of 1.9% while the projected figures reached 173 million by 2010 and 221 million by 2025. These estimates suggest that the country would slip below the limit of 1000m3 of water per capita per year from 2010 onwards. The situation could get worse in areas situated outside Indus basin where annual average is already below 1000m3 per head. Water Shortage Water shortage is yet another major obstacle to the development of the country in terms of food security, economic development and industrialization. Even if an improvement of 50% in agricultural productivity with respect to the use of water is considerable, this however remains an unrealistic target. -

Crisis Response Bulletin V3I1.Pdf
IDP IDP IDP CRISIS RESPONSE BULLETIN January 02, 2017 - Volume: 3, Issue: 01 IN THIS BULLETIN HIGHLIGHTS: English News 03-27 Water reservoirs may reach dead level in two months 03 Dense fog causes closure of motorway, delays flights 04 UNHCR supports free health services to mothers and children in 06 Natural Calamities Section 03-07 Quetta Safety and Security Section 08-22 Pakistan 7th most ‘climate change venerable country’ 07 Public Services Section 23-27 Thank you Raheel Sharif: From 3rd most dangerous city in the 08 world, Karachi falls to 31st place in 3 years Maps 28-29 Pakistan faces India sponsored terrorism: FO 09 Military courts head towards completion of term 10 Crime rate decreased significantly in 2016 15 Urdu News 41-30 3500 ‘terrorists’ killed in 2016, says army report 15 US sanctions seven Pakistani entities 18 Natural Calamities Section 41-40 Over 450,000 fake CNICs blocked in three years: Ch Nisar 19 Army to facilitate local population in health, edu sectors: Brig Tahir23 Safety and Security section 39-36 HEC decides to launch online system for degree verification 23 Public Service Section 35-30 ‘WAPDA registered 5.7% rise in hydropower generation’ 24 MAPS DROUGHT SITUATION MAP - PAKISTAN DROUGHT HIT IN THAR Drought Hit in Thar Legend Outbreak of Waterborne Diseases (from Jan,2016 to Dec, 2016) G Basic Health Unit Government & Private Health Facility ÷Ó Children Hospital Health Facility Government Private Total Sanghar Basic Health Unit 21 0 21 G Dispensary At least nine more infants died due to malnutrition and outbreak of the various diseases in Thar during that last two Children Hospital 0 1 1 days, raising the toll to 476 this year.With the death of nine more children the toll rose to 476 during past 12 months Dispensary 12 0 12 "' District Headquarter Hospital of the outgoing year, said health officials. -
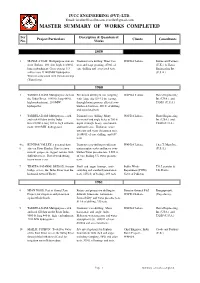
Master Summary of Works Completed
IVCC ENGINEERING (PVT) LTD. Email: [email protected], [email protected] MASTER SUMMARY OF WORKS COMPLETED Ser Description & Quantum of Project Particulars Clients Consultants No. Works 1959 1 MANGLA DAM. Multipurpose dam on Diamond core drilling. Water loss WAPDA Lahore. Binnie and Partners river Jhelum. 380 feet high, 11,000 ft tests and stage grouting. 470 ft. of (U.K.) & Harza long embankment. Gross storage 5.3 core-drilling and associated tests. Engineering Int. million acre ft.1400MW hydropower. (U.S.A.) Work in association with Geostrazivanja (Yugoslavia). 1960 2 TARBELA DAM. Multipurpose dam on Percussion drilling & soil sampling WAPDA Lahore. Harza Engineering the Indus River. 9,000 ft. long 480 ft. with large dia (20 -12 in) casing, Int. (USA.) and high embankment. 2100MW through heterogeneous alluvial over- TAMS (U.S.A.) hydropower. burden & boulders. 800 ft. of drilling and associated tests. 3 TARBELA DAM. Multipurpose earth Diamond core-drilling. Many WAPDA Lahore. Harza Engineering and rock fill dam on the Indus horizontal and angle holes to 700 ft. Int. (USA.) and River.9,000 ft long 480 ft. high embank- depth through heavy overburden TAMS (U.S.A.) ment. 2100 MW hydropower. and hard rocks. Elaborate water pressure and water absorption tests. 16,490 ft. of core drilling and 657 tests. 4 to KUNHAR VALLEY. 3 potential dam Diamond core-drilling in different WAPDA Lahore. Chas.T. Main Inc., 6 sites on River Kunhar. Sites in steep metamorphic rocks and heavy over- (U.S.A.) narrow gorges, in rugged terrain, with burden.Water pressure tets. 3,000 ft. -

Impacts of Landuse Changes on Runoff Generation In
Sci.Int.(Lahore),27(4),3185-3191,2015 ISSN 1013-5316; CODEN: SINTE 8 3185 IMPACTS OF LANDUSE CHANGES ON RUNOFF GENERATION IN SIMLY WATERSHED *Tanveer Abbas1, Ghulam Nabi2, Muhammad Waseem Boota1 Fiaz Hussain1, Muhammad Faisal1 and Haseeb Ahsan1 1 Centre of Excellence in Water Resources Engineering, University Of Engineering and Technology Lahore 2 Centre of Excellence in Water Resources Engineering, University Of Engineering and Technology Lahore *Corresponding Author’s email: [email protected], [email protected] ABSTRACT: Landuse change has significant impacts on hydrologic processes in a watershed. Assessment of the impacts of landuse changes is essential for watershed management and sustainable development. In this study, a soil and water assessment tool (SWAT) model was used to assess the landuse changes impacts on runoff generation in Simly dam watershed located 30 km east of Islamabad, Pakistan with a catchment area of 156 km2.The simulation was performed for three time periods 1991-92, 2000-01, 2009-10 on a monthly time step. The model was calibrated using the auto-calibration tool SWAT- CUP. Model predicted surface runoff volumes with Nash Sutcliffe Efficiency (NSE) ranges from 0.81 to 0.83. The results of the scenarios revealed that expansion of urban areas from 5% to 10% indicated 1.2% and 0.2% increase in the surface runoff and water yield respectively, expansion of forests areas from 46% to 66% indicated 9.3% and 2.5% decrease in the surface runoff and water yield respectively and deforestation of the catchment from 46% to 36% indicated 6.7% and 2.2% increase in the surface runoff and water yield respectively. -

Land and Water Resources of Pakistan— a Critical Assessment
©The Pakistan Development Review 46 : 4 Part II (Winter 2007) pp. 911–937 Land and Water Resources of Pakistan— A Critical Assessment * SHAHID AHMAD 1. RESOURCE AVAILABILITY 1.1. Land Resources The country’s geographical area is 79.61 million hectares (mha), excluding the Northern Areas of Pakistan. Out of this, only 72 percent area has been reported indicating a major limitation that 28 percent area is not yet surveyed for land use classification. The reported area is further classified into four major classes: (a) forest area of 4.02 mha; (b) area not available for cultivation of 22.88 mha; (c) culturable waste of 8.12 mha; and (d) cultivated area of 22.05 mha. Out of the reported area, around 8.1 mha are available for future agriculture and other uses, if water is made available. If rest of the area (28 percent) is also surveyed then one can have better picture of country’s land resources (Table 1). Table 1 Land Resources Classification of Pakistan Area Type of Land Use (mha) (%) Geographical Area 79.61 100.00 Total Reported Area 57.07 71.7 Forest Area 4.02 5.1 Area Not Available for Cultivation 22.88 28.7 Culturable Waste 8.12 10.2 Area Cultivated for Agriculture 22.05 27.7 Source: Pakistan (2006). 1.2. Water Resources 1.2.1. Precipitation and Aridity Country’s mean annual rainfall varies from <100 mm in parts of Balochistan and Sindh provinces to >1500 mm in foothills and northern mountains. In the Northern Areas at altitudes of above 5000 m, the snowfall exceeds 5000 mm and provides the largest Shahid Ahmad <[email protected]> is Project Coordinator, Supporting Public Resource Management in Balochistan, Balochistan Resource Management Programme, Asian Development Bank, and Departments of Finance and Irrigation and Power, Quetta. -

Developments in and Around Murree Hills
IUCN Pakistan Rapid Environmental Appraisal of Developments in and Around Murree Hills May 2005 Five Year Plan 2005-2010 1 IUCN’s Input to Brown Sector Component of Environment Chapter Contents Acronyms and Abbreviations………………………………………………………………………………………..ii Executive Summary ......................................................................................................................................iii 1. Introduction ......................................................................................................................................1 2. Developments in Murree Hills ..........................................................................................................1 2.1 Rawal Lake: .................................................................................................................................2 3. Legal Action .....................................................................................................................................2 4. New Murree......................................................................................................................................2 4.1 New Murree Development Authority (NMDA):.............................................................................3 4.2 Key Issues related to New Murree: .............................................................................................4 4.2.1 Protected Forest:.....................................................................................................................4 4.2.2 Geological -
NDMA Monsoon 2020 Daily Situation Report No
Page 1 of 9 MOST IMMEDIATE / BY FAX F.1 (E)/2020-NDMA (MW/SITREP) Government of Pakistan Prime Minister's Office National Disaster Management Authority NDMA ISLAMABAD Dated: 27 August 2020 Subject. NDMA Monsoon 2020 Daily Situation Report No 063 NDMA Monsoon 2020 Daily Situation Report No 063 covering period from 26 August 2020 1300 hours to 27 August 2020 1300 hours is enclosed for information / necessary action, please. Lieutenant Colonel For Chairman NDMA (Muhammad Ala Ud Din) Tel. 051-9087874 Fax No: 9205086 To: Secretary to Prime Minister, Prime Minister's Office, Islamabad Military Secretary to Prime Minister, Prime Minister's House, Islamabad Cc: Director General, Pakistan Meteorological Department, Islamabad Director General, Frontier Works Organization, Rawalpindi Chairman, Federal Flood Commission, Islamabad Chairman, National Highway Authority, Islamabad Joint Commissioner, Pakistan Commission for Indus Water, Islamabad Chief Meteorologist, Flood Forecasting Division, Lahore Chief Engineer, Tarbela Dam Chief Engineer, Mangla Dam Director General, NHEPRN, Islamabad Director General, PDMA Balochistan, Quetta Director General, PDMA Khyber Pakhtunkhwa, Peshawar Director General, PDMA Punjab, Lahore Director General, PDMA Sindh, Karachi Director General, SDMA AJ&K, Muzaffarabad Director General, GBDMA, Gilgit Joint Crisis Management Cell, Joint Staff Headquarters, Rawalpindi Military Operation Directorate (MO-4), GHQ, Rawalpindi Arms Branch, Engineers Directorate, GHa, Rawalpindi Director (SOF &Marines), Naval Headquarters, Islamabad Director (Ops), Air Headquarters, Islamabad Deputy Commissioner, Islamabad ID: APS to Member (Ops), NDMA APS to Member (A&F), NDMA APS to Member (DRR), NDMA Director (Logistics), NDMA Director (R&R), NDMA Director (lImplementation), NDMA Director (P&IC), NDMA Director (Admin), NDMA Director (Finance), NDMA Director (Media &IT),. -

Morphological and Hydrological Responses of Soan and Khad Rivers 269
Morphological and hydrological responses of Soan and Khad rivers 269 MORPHOLOGICAL AND HYDROLOGICAL RESPONSES OF SOAN AND KHAD RIVERS TO IMPERVIOUS LAND USE IN DISTRICT MURREE, PAKISTAN Fida Hussain, Abdul Khaliq, M. Irfan Ashraf, Irshad A. Khan* ABSTRACT The study was conducted during 2005 to 2009 in District Muree, Pakistan. Rapid degradation of watersheds due to soil erosion, deforestation, urbanization is the critical issue in high hill areas. Housing and infrastructure development, being the top priority of government is also impacting the health of watersheds; resultantly our dams are losing their capacity due to increased sedimentation. The study aimed at assessing the current magnitude and distribution of development in two important watersheds of Punjab. The assessment was carried out by correlating infrastructure development with soil erosion and regime of water in the channels. Impervious land use in Simly watershed is increasing at a pace of 1.91 % per year and presently it is 13.23 % of the total area. It has also been determined that at current pace of development, 16.84 % of the area in Simly will be under impervious land use by 2020. Based on the land use of the area, different channels in Simly stream system were categorized into urbanizing, agriculture and forest and main channel. The study revealed that the soil erosion is more in areas under impervious land use or where the land is disturbed due to developmental activities. The sediment load in different categories of channels was studied. It was found that sediment load in the urbanizing water channel was highest (26.03 g/l) followed by agriculture (8.86 g/l), and forest was the least (1.73 g/l) contributor of sediment in the channels. -

PAKISTAN ENGINEERING CONGRESS 64TH ANNUAL SESSION the EXECUTIVE COUNCIL for the 64TH SESSION (1989 – 90) PRESIDENT Engr
Pakistan Engineering Congress in Retrospect (1912 – 2012) Centenary Celebration 573 PAKISTAN ENGINEERING CONGRESS 64TH ANNUAL SESSION THE EXECUTIVE COUNCIL FOR THE 64TH SESSION (1989 – 90) PRESIDENT Engr. Shams ul Mulk VICE PRESIDENTS 1. Engr. Muhammad Aslam Chohan 9. Engr. Akhtar Ali Shah 2. Engr. Rana Khursheed Anver 10. Engr. Rana Muhammad Saeed Ahmad Khan 3. Engr. Aftab Ahmad Khan 11. Engr. Abdur Razik Khan 4. Engr. M. R. Shad 12. Engr. Faqir Ahmad Paracha 5. Engr. Abdul Rehman Baloch 13. Engr. Muzammil Hussain Qureshi 6. Engr. Ch. Muhammad Rashid Khan 14. Engr. Khalid Jalil 7. Engr. S. N. H. Mashhadi 15. Engr. Dr. Ikram ul Haq Khan 8. Engr. Zafar-ullah Khan OFFICE BEARERS 1. Engr. Dr. Izhar-ul-Haq Secretary 2. Engr. Iftikhar-ul-Haq Joint Secretary 3. Engr. Brig. Khurshid Ghias Ahmad Business Manager 4. Engr. Haroon Rashid Toosy Publicity Secretary 5. Engr. Ch. Haider Ali Treasurer 6. Engr. Mian Fazal Ahmad Chief Editor / Engg. News 7. Engr. Mrs. Naheed Ghazanfar Addl. Chief Editor / Engg. News EXECUTIVE COUNCIL MEMBERS 1. Engr. Mohammad Ikhramullah 24. Engr. Safdar Hussain Khan 2. Engr. Abdul Vakil Malik 25. Engr. M. M. Khan 3. Engr. Sheikh Muhammad Ashraf 26. Engr. Khalil-ur-Rehman 4. Engr. Mian Sikandar Hayat 27. Engr. Ikramul Haq 5. Engr. Ch. Abdul Khalid 28. Engr. Imran Maqsud 6. Engr. Izhar Irshad Chaudhry 29. Engr. Abdul Hamid Arif 7. Engr. Muhammad Siddiq 30. Engr. Mian Muhammad Amin 8. Engr. Ashfaq Ahmed Qureshi 31. Engr. Muhammad Amin Khan Mazhar 9. Engr. A. H. Zaidi 32. Engr. Rashid Ahmad Beg 10. Engr. -

The Indus Equation 2 Introduction CHAPTER 1 Overview of Pakistan’S Water Resources
C-306, Montana, Lokhandwala Complex Andheri West, Mumbai 400 053, India Email: [email protected] www.strategicforesight.com Project Advice Ilmas Futehally Authors Gitanjali Bakshi Sahiba Trivedi Creative Preeti Rathi Motwani Copyright © Strategic Foresight Group, 2011 Permission is hereby granted to quote or reproduce from this report with due credit to Strategic Foresight Group Processed by MadderRed, Mumbai FOREWORD Strategic Foresight Group has been a consistent advocate of reason in relations between India and Pakistan. It has recognised water as a critical determinant of peace and development in many parts of the world. This paper brings together these two strands in our work. It will be in order to recall some of the earlier work done by Strategic Foresight Group to urge rationality in India- Pakistan relations. In 2004, we published the first ever comprehensive assessment of Cost of Conflict between India-Pakistan in a report with this title. In 2005 we published The Final Settlement where we strongly argued that integrated water development would need to be a part of long-lasting solution between the two countries. Since then we have been regularly advocating a pragmatic approach for India and Pakistan to foster cooperation and move ahead to enable social and economic development of their people, instead of wasting precious resources on terrorism, counter terrorism and an arms race. We initiated work on water on the advice of an international conference onResponsibility to the Future, which was co-hosted by SFG with the United Nations Global Compact, inaugurated by the President of India and attended by delegates from 25 countries in June 2008.