Cortical Thickness of the Dorsolateral Prefrontal Cortex Predicts Strategic Choices in Economic Games
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
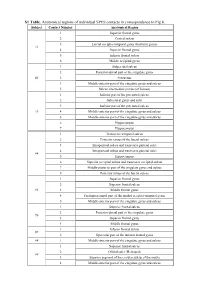
S1 Table. Anatomical Regions of Individual SPES Contacts in Correspondence to Fig 8
S1 Table. Anatomical regions of individual SPES contacts in correspondence to Fig 8. Subject Contact Number Anatomical Region 1 Superior frontal gyrus 2 Central sulcus 3 Lateral occipito-temporal gyrus (fusiform gyrus) #1 4 Superior frontal gyrus 5 Inferior frontal sulcus 6 Middle occipital gyrus 1 Subparietal sulcus 2 Posterior-dorsal part of the cingulate gyrus #2 3 Precuneus 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Sulcus intermedius primus (of Jensen) 1 Inferior part of the precentral sulcus 2 Subcentral gyrus and sulci 3 Inferior part of the precentral sulcus #3 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Middle-anterior part of the cingulate gyrus and sulcus 6 Hippocampus 7 Hippocampus 1 Transverse temporal sulcus 2 Posterior ramus of the lateral sulcus 3 Intraparietal sulcus and transverse parietal sulci 4 Intraparietal sulcus and transverse parietal sulci #4 5 Hippocampus 6 Superior occipital sulcus and transverse occipital sulcus 7 Middle-posterior part of the cingulate gyrus and sulcus 8 Posterior ramus of the lateral sulcus 1 Superior frontal gyrus 2 Superior frontal sulcus #5 3 Middle frontal gyrus 4 Parahippocampal part of the medial occipito-temporal gyrus 5 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Posterior-dorsal part of the cingulate gyrus #6 3 Superior frontal gyrus 4 Middle frontal gyrus 1 Inferior frontal sulcus #7 2 Opercular part of the inferior frontal gyrus #8 1 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Orbital sulci (H-shaped) #9 3 Superior segment of the circular sulcus of the insula 4 Middle-anterior part of the cingulate gyrus and sulcus . -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

01 05 Lateral Surface of the Brain-NOTES.Pdf
Lateral Surface of the Brain Medical Neuroscience | Tutorial Notes Lateral Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the lateral view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -

1. Lateral View of Lobes in Left Hemisphere TOPOGRAPHY
TOPOGRAPHY T1 Division of Cerebral Cortex into Lobes 1. Lateral View of Lobes in Left Hemisphere 2. Medial View of Lobes in Right Hemisphere PARIETAL PARIETAL LIMBIC FRONTAL FRONTAL INSULAR: buried OCCIPITAL OCCIPITAL in lateral fissure TEMPORAL TEMPORAL 3. Dorsal View of Lobes 4. Ventral View of Lobes PARIETAL TEMPORAL LIMBIC FRONTAL OCCIPITAL FRONTAL OCCIPITAL Comment: The cerebral lobes are arbitrary divisions of the cerebrum, taking their names, for the most part, from overlying bones. They are not functional subdivisions of the brain, but serve as a reference for locating specific functions within them. The anterior (rostral) end of the frontal lobe is referred to as the frontal pole. Similarly, the anterior end of the temporal lobe is the temporal pole, and the posterior end of the occipital lobe the occipital pole. TOPOGRAPHY T2 central sulcus central sulcus parietal frontal occipital lateral temporal lateral sulcus sulcus SUMMARY CARTOON: LOBES SUMMARY CARTOON: GYRI Lateral View of Left Hemisphere central sulcus postcentral superior parietal superior precentral gyrus gyrus lobule frontal intraparietal sulcus gyrus inferior parietal lobule: supramarginal and angular gyri middle frontal parieto-occipital sulcus gyrus incision for close-up below OP T preoccipital O notch inferior frontal cerebellum gyrus: O-orbital lateral T-triangular sulcus superior, middle and inferior temporal gyri OP-opercular Lateral View of Insula central sulcus cut surface corresponding to incision in above figure insula superior temporal gyrus Comment: Insula (insular gyri) exposed by removal of overlying opercula (“lids” of frontal and parietal cortex). TOPOGRAPHY T3 Language sites and arcuate fasciculus. MRI reconstruction from a volunteer. central sulcus supramarginal site (posterior Wernicke’s) Language sites (squares) approximated from electrical stimulation sites in patients undergoing operations for epilepsy or tumor removal (Ojeman and Berger). -

Cortical Parcellation Protocol
CORTICAL PARCELLATION PROTOCOL APRIL 5, 2010 © 2010 NEUROMORPHOMETRICS, INC. ALL RIGHTS RESERVED. PRINCIPAL AUTHORS: Jason Tourville, Ph.D. Research Assistant Professor Department of Cognitive and Neural Systems Boston University Ruth Carper, Ph.D. Assistant Research Scientist Center for Human Development University of California, San Diego Georges Salamon, M.D. Research Dept., Radiology David Geffen School of Medicine at UCLA WITH CONTRIBUTIONS FROM MANY OTHERS Neuromorphometrics, Inc. 22 Westminster Street Somerville MA, 02144-1630 Phone/Fax (617) 776-7844 neuromorphometrics.com OVERVIEW The cerebral cortex is divided into 49 macro-anatomically defined regions in each hemisphere that are of broad interest to the neuroimaging community. Region of interest (ROI) boundary definitions were derived from a number of cortical labeling methods currently in use. Protocols from the Laboratory of Neuroimaging at UCLA (LONI; Shattuck et al., 2008), the University of Iowa Mental Health Clinical Research Center (IOWA; Crespo-Facorro et al., 2000; Kim et al., 2000), the Center for Morphometric Analysis at Massachusetts General Hospital (MGH-CMA; Caviness et al., 1996), a collaboration between the Freesurfer group at MGH and Boston University School of Medicine (MGH-Desikan; Desikan et al., 2006), and UC San Diego (Carper & Courchesne, 2000; Carper & Courchesne, 2005; Carper et al., 2002) are specifically referenced in the protocol below. Methods developed at Boston University (Tourville & Guenther, 2003), Brigham and Women’s Hospital (McCarley & Shenton, 2008), Stanford (Allan Reiss lab), the University of Maryland (Buchanan et al., 2004), and the University of Toyoma (Zhou et al., 2007) were also consulted. The development of the protocol was also guided by the Ono, Kubik, and Abernathy (1990), Duvernoy (1999), and Mai, Paxinos, and Voss (Mai et al., 2008) neuroanatomical atlases. -

Not for Reprintlaboratory INVESTIGATION Anterior Peri-Insular Quadrantotomy: a Cadaveric White Matter Dissection Study
Authors: Please review all authors’ names, academicLow-resolution degrees, affiliations, and PDF contributions, as well as the Disclosure, MS# 19-472, read before authors for spelling and accuracy. Not for reprintLABORATORY INVESTIGATION Anterior peri-insular quadrantotomy: a cadaveric white matter dissection study *Pablo Gonzalez-Lopez, MD, PhD,1 Giulia Cossu, MD,2 Etienne Pralong, MD,2 Matias Baldoncini, MD,3 Mahmoud Messerer, MD, MSc,2,4 and Roy Thomas Daniel, MD, MCh2,4 1Department of Neurosurgery, Hospital General Universitario Alicante, Spain; 2Department of Neurosurgery, University Hospital of Lausanne, Switzerland; 3Department of Neurological Surgery, San Fernando Hospital, Buenos Aires, Argentina; and 4Faculty of Medicine and Biology, University of Lausanne, Switzerland OBJECTIVE Anterior quadrant disconnection represents a safe surgical option in well-selected pediatric patients with a large frontal lobe lesion anterior to the motor cortex. The understanding of the anatomy of the white matter tracts con- necting the frontal lobe with the rest of the cerebrum forms the basis of a safe and successful disconnective surgery. The authors explored and illustrated the relevant white matter tracts sectioned during each surgical step using fiber dissection techniques. METHODS Five human cadaveric hemispheres were dissected to illustrate the frontal connections in the 3 planes. The dissections were performed from lateral to medial, medial to lateral, and ventral to dorsal to describe the various tracts sectioned during the 4 steps of this surgery, namely the anterior suprainsular window, intrafrontal disconnection, anterior callosotomy, and frontobasal disconnection. RESULTS At the beginning of each surgical step, the U fibers were cut. During the anterior suprainsular window, the superior longitudinal fasciculus (SLF), the uncinate fasciculus, and the inferior fronto-occipital fasciculus (IFOF) were vi- sualized and sectioned, followed by sectioning of the anterior limb of the internal capsule. -

Normal Cortical Anatomy
Normal Cortical Anatomy MGH Massachusetts General Hospital Harvard Medical School NORMAL CORTICAL ANATOMY • Sagittal • Axial • Coronal • The Central Sulcus NP/MGH Sagittal Neuroanatomy NP/MGH Cingulate sulcus Superior frontal gyrus Marginal ramus of Cingulate sulcus Cingulate gyrus Paracentral lobule Superior parietal lobule Parietooccipital sulcus Cuneus Calcarine sulcus Lingual gyrus Subcallosal gyrus Gyrus rectus Fastigium, fourth ventricle NP/MGH Superior frontal gyrus Cingulate sulcus Precentral gyrus Marginal ramus of Cingulate gyrus Central sulcus Cingulate sulcus Superior parietal lobule Precuneus Parietooccipital sulcus Cuneus Calcarine sulcus Frontomarginal gyrus Lingual gyrus Caudothallamic groove Gyrus rectus NP/MGH Precentral sulcus Central sulcus Superior frontal gyrus Marginal ramus of Corona radiata Cingulate sulcus Superior parietal lobule Precuneus Parietooccipital sulcus Calcarine sulcus Inferior occipital gyrus Lingual gyrus NP/MGH Central sulcus Superior parietal lobule Parietooccipital sulcus Frontopolar gyrus Frontomarginal gyrus Superior occipital gyrus Middle occipital gyrus Medial orbital gyrus Lingual gyrus Posterior orbital gyrus Inferior occipital gyrus Inferior temporal gyrus Temporal horn, lateral ventricle NP/MGH Central sulcus Superior Temporal gyrus Middle Temporal gyrus Inferior Temporal gyrus NP/MGH Central sulcus Superior parietal gyrus Inferior frontal gyrus Frontomarginal gyrus Anterior orbital gyrus Superior occipital gyrus Middle occipital Posterior orbital gyrus gyrus Superior Temporal gyrus Inferior -

Dynamic Mapping of Human Cortical Development During Childhood Through Early Adulthood
Dynamic mapping of human cortical development during childhood through early adulthood Nitin Gogtay*†, Jay N. Giedd*, Leslie Lusk*, Kiralee M. Hayashi‡, Deanna Greenstein*, A. Catherine Vaituzis*, Tom F. Nugent III*, David H. Herman*, Liv S. Clasen*, Arthur W. Toga‡, Judith L. Rapoport*, and Paul M. Thompson‡ *Child Psychiatry Branch, National Institutes of Mental Health, National Institutes of Health, Bethesda, MD 20892; and ‡Laboratory of Neuro Imaging, Department of Neurology, University of California School of Medicine, Los Angeles, CA 90095-1769 Communicated by Leslie G. Ungerleider, National Institutes of Health, Bethesda, MD, April 15, 2004 (received for review January 7, 2004) We report the dynamic anatomical sequence of human cortical temporal cortex, which contains association areas that integrate gray matter development between the age of 4–21 years using information from several sensory modalities, matured last. Fur- quantitative four-dimensional maps and time-lapse sequences. thermore, the maturation of the cortex also appeared to follow Thirteen healthy children for whom anatomic brain MRI scans were the evolutionary sequence in which these regions were created. obtained every 2 years, for 8–10 years, were studied. By using models of the cortical surface and sulcal landmarks and a statistical Methods model for gray matter density, human cortical development could Subjects. Sample demographics are shown in Table 1. All subjects be visualized across the age range in a spatiotemporally detailed were recruited from the community for an ongoing National time-lapse sequence. The resulting time-lapse ‘‘movies’’ reveal that Institute of Mental Health study of human brain development (i) higher-order association cortices mature only after lower-order (20). -

Mirror Neurons for Execution, Observation, and Imagery ⁎ Flavia Filimon,A, Jonathan D
www.elsevier.com/locate/ynimg NeuroImage 37 (2007) 1315–1328 Human cortical representations for reaching: Mirror neurons for execution, observation, and imagery ⁎ Flavia Filimon,a, Jonathan D. Nelson,a,b Donald J. Hagler,a and Martin I. Serenoa aDepartment of Cognitive Science, University of California, San Diego, 9500 Gilman Dr. #0515, La Jolla, CA 92093-0515, USA bComputational Neurobiology Laboratory, Salk Institute, La Jolla, CA, USA Received 5 April 2007; revised 27 May 2007; accepted 8 June 2007 Available online 18 June 2007 We used functional magnetic resonance imaging (fMRI) to map the Electrophysiological studies in macaques have identified several cortical representations of executed reaching, observed reaching, and frontal areas involved in hand action representations (Preuss et al., imagined reaching in humans. Whereas previous studies have mostly 1996; Muakkassa and Strick, 1979; Matelli and Luppino, 2001; examined hand actions related to grasping, hand–object interactions, Rizzolatti et al., 1988). For instance, both dorsal premotor cortex or local finger movements, here we were interested in reaching only (i.e. (PMd, or F2 and F7) and ventral premotor cortex (PMv, or F4 and the transport phase of the hand to a particular location in space), F5) contain arm/hand representations that are specific to certain without grasping. We hypothesized that mirror neuron areas specific to reaching-related representations would be active in all three conditions. motor actions, such as grasping or reaching (Matelli and Luppino, An overlap between executed, observed, and imagined reaching 2001). More specifically, neurons that respond to both hand action activations was found in dorsal premotor cortex as well as in the execution (e.g. -

Reorganization of the Neurobiology of Language After Sentence Overlearning
Sentence overlearning 1 Reorganization of the neurobiology of language after sentence overlearning 1* 1,2 3 1 4 Jeremy I Skipper , Sarah Aliko , Stephen Brown , Yoon Ju Jo , Serena Lo , Emilia 5 1,6 Molimpakis and Daniel R Lametti 1 E xperimental Psychology, University College London, UK 2 L ondon Interdisciplinary Biosciences Consortium, University College London, UK 3 N atural Sciences, University College London, UK 4 S peech and Language Sciences, University College London, UK 5 W ellcome Centre for Human Neuroimaging, University College London, UK 6 D epartment of Psychology, Acadia University, Nova Scotia, Canada * C orresponding author, [email protected] Supplementary Material Figure Captions Figure S1. Additional novel listening linear mixed-effects model results. General linear test contrasting novel sentences from session one (blues) and two (reds) in the left (LH) and right hemispheres (RH) presented on lateral (top) and medial (bottom) surface views. The colour bar represents z-scores and the images are thresholded at an alpha (α) level of p < .01, corrected for multiple comparisons. Figure S2. Additional overlearned sentence listening linear mixed-effects model results. A) Session one general linear test (GLT) for sentence; B) Session two GLT for sentence; C) Direct contrast of session one sentences (blues) with session two sentences (reds). The top two rows are the left hemisphere (LH) while the bottom two are the right hemispheres (RH) lateral and medial surface views. The colour bar represents z-scores and the images are thresholded at an alpha (α) level of p < .01, corrected for multiple comparisons. Figure S3. Additional overlearned minus novel sentence listening linear mixed-effects model results. -

Schaer K., Jahn G., Lotze M. (2012) Fmri-Activation During Drawing A
Behavioural Brain Research 233 (2012) 209–216 Contents lists available at SciVerse ScienceDirect Behavioural Brain Research j ournal homepage: www.elsevier.com/locate/bbr Research report fMRI-activation during drawing a naturalistic or sketchy portrait a b a,∗ K. Schaer ,G.Jahn , M. Lotze a Functional Imaging Unit, Center for Diagnostic Radiology and Neuroradiology, University of Greifswald, Greifswald, Germany b Department of Psychology, University of Greifswald, Greifswald, Germany h i g h l i g h t s We used fMRI to measure 20 naive subjects during drawing a portrait. Participants were able to track their drawing online. We identified three important circuits specific for the process of portrait drawing. Circuits where: face perception, location encoding, and continuous feedback processes. Representations involved: fusiform gyrus, precuneus, parietal sulcus, and cerebellum. a r t i c l e i n f o a b s t r a c t Article history: Neural processes for naturalistic drawing might be discerned into object recognition and analysis, atten- Received 8 March 2012 tion processes guiding eye hand interaction, encoding of visual features in an allocentric reference frame, Received in revised form 3 May 2012 a transfer into the motor command and precise motor guidance with tight sensorimotor feedback. Cere- Accepted 8 May 2012 bral representations in a real life paradigm during naturalistic drawing have sparsely been investigated. Available online 15 May 2012 Using a functional Magnetic Resonance Imaging (fMRI) paradigm we measured 20 naive subjects during drawing a portrait from a frontal face presented as a photograph. Participants were asked to draw the Keywords: portrait in either a naturalistic or a sketchy characteristic way. -

Subregions of the Human Superior Frontal Gyrus and Their Connections
NeuroImage 78 (2013) 46–58 Contents lists available at SciVerse ScienceDirect NeuroImage journal homepage: www.elsevier.com/locate/ynimg Subregions of the human superior frontal gyrus and their connections Wei Li a,1, Wen Qin a,1, Huaigui Liu a, Lingzhong Fan b, Jiaojian Wang b, Tianzi Jiang b,⁎, Chunshui Yu a,⁎⁎ a Department of Radiology, Tianjin Medical University General Hospital, Tianjin 300052, PR China b LIAMA Center for Computational Medicine, National Laboratory of Pattern Recognition, Institute of Automation, Chinese Academy of Sciences, Beijing 100190, PR China article info abstract Article history: The superior frontal gyrus (SFG) is located at the superior part of the prefrontal cortex and is involved in a variety Accepted 5 April 2013 of functions, suggesting the existence of functional subregions. However, parcellation schemes of the human SFG Available online 13 April 2013 and the connection patterns of each subregion remain unclear. We firstly parcellated the human SFG into the anteromedial (SFGam), dorsolateral (SFGdl), and posterior (SFGp) subregions based on diffusion tensor Keywords: tractography. The SFGam was anatomically connected with the anterior and mid-cingulate cortices, which are Diffusion tensor imaging critical nodes of the cognitive control network and the default mode network (DMN). The SFGdl was connected Superior frontal gyrus Tractography with the middle and inferior frontal gyri, which are involved in the cognitive execution network. The SFGp was Parcellation connected with the precentral gyrus, caudate, thalamus, and frontal operculum, which are nodes of the motor Resting-state control network. Resting-state functional connectivity analysis further revealed that the SFGam was mainly Fingerprint correlated with the cognitive control network and the DMN; the SFGdl was correlated with the cognitive execution network and the DMN; and the SFGp was correlated with the sensorimotor-related brain regions.