International Nonproprietary Names for Pharmaceutical Substances (INN)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Use of Stems in the Selection of International Nonproprietary Names (INN) for Pharmaceutical Substances" WHO/EMP/RHT/TSN/2018.1
INN Working Document 19.450 04/02/2019 Addendum1 to "The use of stems in the selection of International Nonproprietary names (INN) for pharmaceutical substances" WHO/EMP/RHT/TSN/2018.1 Programme on International Nonproprietary Names (INN) Technologies Standards and Norms (TSN) Regulation of Medicines and other health technologies (RHT) World Health Organization, Geneva © World Health Organization 2019 - All rights reserved. The contents of this document may not be reviewed, abstracted, quoted, referenced, reproduced, transmitted, distributed, translated or adapted, in part or in whole, in any form or by any means, without explicit prior authorization of the WHO INN Programme. This document contains the collective views of the INN Expert Group and does not necessarily represent the decisions or the stated policy of the World Health Organization. Addendum1 to "The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances" - WHO/EMP/RHT/TSN/2018.1 1 This addendum is a cumulative list of all new stems selected by the INN Expert Group since the publication of "The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances" 2018. ------------------------------------------------------------------------------------------------------------ -calcet/-calcet- calcium-sensing receptors (CaSR) agonists cinacalcet (88), etelcalcetide (112), evocalcet (113), tecalcet (87), upacicalcet (118) ------------------------------------------------------------------------------------------------------------ -

Effects of Serotonin in the Hippocampus: How Ssris and Multimodal Antidepressants Might Regulate Pyramidal Cell Function
CNS Spectrums (2016), 21, 143–161. © Cambridge University Press 2015. The online version of this article is published within an Open Access environment subject to the conditions of the Creative Commons Attribution-NonCommercial-ShareAlike licence <http://creativecommons.org/licenses/by-nc-sa/3.0/>. The written permission of Cambridge University Press must be obtained for commercial re-use. doi:10.1017/S1092852915000425 REVIEW ARTICLE Effects of serotonin in the hippocampus: how SSRIs and multimodal antidepressants might regulate pyramidal cell function Elena Dale,1* Alan L. Pehrson,1 Theepica Jeyarajah,1 Yan Li,1 Steven C. Leiser,1 Gennady Smagin,1 Christina K. Olsen,2 and Connie Sanchez1 1 Lundbeck Research USA, Paramus, New Jersey, USA 2 Lundbeck DK, Copenhagen-Valby, Denmark The hippocampus plays an important role in emotional and cognitive processing, and both of these domains are affected in patients with major depressive disorder (MDD). Extensive preclinical research and the notion that modulation of serotonin (5-HT) neurotransmission plays a key role in the therapeutic efficacy of selective serotonin reuptake inhibitors (SSRIs) support the view that 5-HT is important for hippocampal function in normal and disease- like conditions. The hippocampus is densely innervated by serotonergic fibers, and the majority of 5-HT receptor subtypes are expressed there. Furthermore, hippocampal cells often co-express multiple 5-HT receptor subtypes that can have either complementary or opposing effects on cell function, adding to the complexity of 5-HT neurotransmission. Here we review the current knowledge of how 5-HT, through its various receptor subtypes, modulates hippocampal output and the activity of hippocampal pyramidal cells in rodents. -

Stems for Nonproprietary Drug Names
USAN STEM LIST STEM DEFINITION EXAMPLES -abine (see -arabine, -citabine) -ac anti-inflammatory agents (acetic acid derivatives) bromfenac dexpemedolac -acetam (see -racetam) -adol or analgesics (mixed opiate receptor agonists/ tazadolene -adol- antagonists) spiradolene levonantradol -adox antibacterials (quinoline dioxide derivatives) carbadox -afenone antiarrhythmics (propafenone derivatives) alprafenone diprafenonex -afil PDE5 inhibitors tadalafil -aj- antiarrhythmics (ajmaline derivatives) lorajmine -aldrate antacid aluminum salts magaldrate -algron alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol combined alpha and beta blockers labetalol medroxalol -amidis antimyloidotics tafamidis -amivir (see -vir) -ampa ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) subgroup: -ampanel antagonists becampanel -ampator modulators forampator -anib angiogenesis inhibitors pegaptanib cediranib 1 subgroup: -siranib siRNA bevasiranib -andr- androgens nandrolone -anserin serotonin 5-HT2 receptor antagonists altanserin tropanserin adatanserin -antel anthelmintics (undefined group) carbantel subgroup: -quantel 2-deoxoparaherquamide A derivatives derquantel -antrone antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine antineoplastics (arabinofuranosyl derivatives) fazarabine fludarabine aril-, -aril, -aril- antiviral (arildone derivatives) pleconaril arildone fosarilate -arit antirheumatics (lobenzarit type) lobenzarit clobuzarit -arol anticoagulants (dicumarol type) dicumarol -

The Use of Stems in the Selection of International Nonproprietary Names (INN) for Pharmaceutical Substances
WHO/PSM/QSM/2006.3 The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances 2006 Programme on International Nonproprietary Names (INN) Quality Assurance and Safety: Medicines Medicines Policy and Standards The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 © World Health Organization 2006 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. -
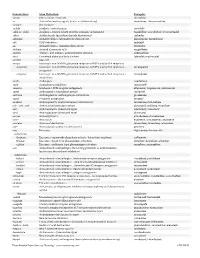
Downloaded from Survive Nursing | Survivenursing.Com V20110426
Generic Stem Stem Definition Examples -abine (see -arabine, -citabine) decitabine -ac Anti-inflammatory agents (acetic acid derivatives) bromfenac; dexpemedolac -acetam See -racetam -actide Synthetic corticotropins seractide -adol or -aldol- Analgesics (mixed opiate receptor agonists/ antagonists) tazadolene; spiradolene; levonantradol -adox Antibacterials (quinoline dioxide derivatives) carbadox -afenone Antiarrhythmics (propafenone derivatives) alprafenone; diprafenone -afil PDE5 inhibitors tadalafil -aj- Antiarrhythmics (ajmaline derivatives) lorajmine -aldrate Antacid aluminum salts magaldrate -algron Alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol Combined alpha and beta blockers labetalol; medroxalol -amivir (see -vir) -ampa Ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) -ampanel Ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) ; becampanel antagonists -ampator Ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) ; forampator modulators -andr- Androgens nandrolone -anib Angiogenesis inhibitors semaxanib -anserin Serotonin 5-HT2 receptor antagonists altanserin; tropanserin; adatanserin -antel Anthelmintics (undefined group) carbantel -antrone Antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine Antineoplastics (arabinofuranosyl derivatives) fazarabine; fludarabine aril-, -aril, -aril- Antiviral (arildone derivatives) pleconaril; arildone; fosarilate -arit Antirheumatics (lobenzarit type) lobenzarit; clobuzarit -arol -

(12) Patent Application Publication (10) Pub. No.: US 2015/0202317 A1 Rau Et Al
US 20150202317A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0202317 A1 Rau et al. (43) Pub. Date: Jul. 23, 2015 (54) DIPEPTDE-BASED PRODRUG LINKERS Publication Classification FOR ALPHATIC AMNE-CONTAINING DRUGS (51) Int. Cl. A647/48 (2006.01) (71) Applicant: Ascendis Pharma A/S, Hellerup (DK) A638/26 (2006.01) A6M5/9 (2006.01) (72) Inventors: Harald Rau, Heidelberg (DE); Torben A 6LX3/553 (2006.01) Le?mann, Neustadt an der Weinstrasse (52) U.S. Cl. (DE) CPC ......... A61K 47/48338 (2013.01); A61 K3I/553 (2013.01); A61 K38/26 (2013.01); A61 K (21) Appl. No.: 14/674,928 47/48215 (2013.01); A61M 5/19 (2013.01) (22) Filed: Mar. 31, 2015 (57) ABSTRACT The present invention relates to a prodrug or a pharmaceuti Related U.S. Application Data cally acceptable salt thereof, comprising a drug linker conju (63) Continuation of application No. 13/574,092, filed on gate D-L, wherein D being a biologically active moiety con Oct. 15, 2012, filed as application No. PCT/EP2011/ taining an aliphatic amine group is conjugated to one or more 050821 on Jan. 21, 2011. polymeric carriers via dipeptide-containing linkers L. Such carrier-linked prodrugs achieve drug releases with therapeu (30) Foreign Application Priority Data tically useful half-lives. The invention also relates to pharma ceutical compositions comprising said prodrugs and their use Jan. 22, 2010 (EP) ................................ 10 151564.1 as medicaments. US 2015/0202317 A1 Jul. 23, 2015 DIPEPTDE-BASED PRODRUG LINKERS 0007 Alternatively, the drugs may be conjugated to a car FOR ALPHATIC AMNE-CONTAINING rier through permanent covalent bonds. -

Recent Development of Non-Peptide Gnrh Antagonists
Review Recent Development of Non-Peptide GnRH Antagonists Feng-Ling Tukun 1, Dag Erlend Olberg 1,2, Patrick J. Riss 2,3,4, Ira Haraldsen 4, Anita Kaass 5 and Jo Klaveness 1,* 1 School of Pharmacy, University of Oslo, 0316 Oslo, Norway; [email protected] (F.-L.T.); [email protected] (D.E.O.) 2 Norsk Medisinsk Syklotronsenter AS, Postboks 4950 Nydalen, 0424 Oslo, Norway; [email protected] 3 Realomics SFI, Department of Chemistry, University of Oslo, 0316 Oslo, Norway 4 Department of neuropsychiatry and psychosomatic medicine, Oslo University Hospital, 4950 Oslo, Norway; [email protected] 5 Betanien Hospital, 3722 Skien, Norway; [email protected] * Correspondence: [email protected]; Tel.: +47-9177-6204 Received: 16 November 2017; Accepted: 4 December 2017; Published: 9 December 2017 Abstract: The decapeptide gonadotropin-releasing hormone, also referred to as luteinizing hormone-releasing hormone with the sequence (pGlu-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH2) plays an important role in regulating the reproductive system. It stimulates differential release of the gonadotropins FSH and LH from pituitary tissue. To date, treatment of hormone-dependent diseases targeting the GnRH receptor, including peptide GnRH agonist and antagonists are now available on the market. The inherited issues associate with peptide agonists and antagonists have however, led to significant interest in developing orally active, small molecule, non-peptide antagonists. In this review, we will summarize all developed small molecule GnRH antagonists along with the most recent clinical data and therapeutic applications. Keywords: GnRH receptor; non-peptide GnRH antagonist 1. -

First Principles and Their Application to Drug Discovery
REVIEWS Drug Discovery Today Volume 17, Numbers 1/2 January 2012 The utilization of the kinetic and thermodynamic signatures of preclinical leads is proving pivotal in their triage and rational optimization towards clinical candidates with maximal in vivo efficacy devoid of adverse events. Reviews KEYNOTE REVIEW Target–drug interactions: first principles and their application to drug discovery 1 1 Sara Nu´n˜ez studied organic Sara Nu´n˜ ez , Jennifer Venhorst and Chris G. Kruse chemistry at the University of Barcelona (Spain) and the Abbott Healthcare Products, 1381 CP Weesp, The Netherlands University of London (UK). She received her Ph.D. in 2003 from the University of Manchester (UK), and thereafter did a In this review, we begin by introducing the basic principles of kinetics postdoc in Biophysics at the and thermodynamics of target–drug binding within the context of Albert Einstein College of Medicine (USA). In 2005, she drug discovery. In addition, we present a meta-analysis of the recent joined Solvay Pharmaceuticals (now Abbott Healthcare) in The Netherlands as a postdoctoral fellow; and in 2008, she literature describing the kinetic and thermodynamic resolution of was promoted to Sr. Computational Medicinal Chemist. At Abbott, she has supported the medicinal chemistry efforts successful clinical candidates with diverse mechanisms of action. for neuroscience drug discovery programs, from target We finish by discussing the best practices in the triage and chemical discovery up to and including clinical proof of principle studies. She has supported more than 15 programs optimization towards clinical candidates with maximal in vivo internationally, and was project manager of the D2-103 Top Institute Pharma innitiative. -

WO 2011/089216 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date t 28 July 2011 (28.07.2011) WO 2011/089216 Al (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 47/48 (2006.01) C07K 1/13 (2006.01) kind of national protection available): AE, AG, AL, AM, C07K 1/1 07 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) Number: International Application DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/EP201 1/050821 HN, HR, HU, ID, J , IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 2 1 January 201 1 (21 .01 .201 1) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (25) Filing Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, (26) Publication Language: English TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 1015 1465. 1 22 January 2010 (22.01 .2010) EP kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, (71) Applicant (for all designated States except US): AS- ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, CENDIS PHARMA AS [DK/DK]; Tuborg Boulevard TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, 12, DK-2900 Hellerup (DK). -

Nonsteroidal Anti-Inflammatory Drugs for Dysmenorrhoea (Review)
Cochrane Database of Systematic Reviews Nonsteroidal anti-inflammatory drugs for dysmenorrhoea (Review) Marjoribanks J, Ayeleke RO, Farquhar C, Proctor M Marjoribanks J, Ayeleke RO, Farquhar C, Proctor M. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No.: CD001751. DOI: 10.1002/14651858.CD001751.pub3. www.cochranelibrary.com Nonsteroidal anti-inflammatory drugs for dysmenorrhoea (Review) Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 SUMMARY OF FINDINGS FOR THE MAIN COMPARISON . ..... 4 BACKGROUND .................................... 5 OBJECTIVES ..................................... 6 METHODS ...................................... 6 Figure1. ..................................... 8 Figure2. ..................................... 10 Figure3. ..................................... 12 RESULTS....................................... 14 Figure4. ..................................... 16 Figure5. ..................................... 18 Figure6. ..................................... 24 ADDITIONALSUMMARYOFFINDINGS . 25 DISCUSSION ..................................... 26 AUTHORS’CONCLUSIONS . 27 ACKNOWLEDGEMENTS . 27 REFERENCES ..................................... 28 CHARACTERISTICSOFSTUDIES . 40 DATAANDANALYSES. 130 Analysis 1.1. Comparison 1 NSAIDs vs placebo, Outcome 1 Pain relief dichotomous data. 136 -

United States Patent (10) Patent No.: US 7,368,477 B2 Gross Et Al
USOO7368477B2 (12) United States Patent (10) Patent No.: US 7,368,477 B2 Gross et al. (45) Date of Patent: May 6, 2008 (54) BENZOFURANYLALKANAMINE 6,419,958 B2 7/2002 Sherman et al. DERVATIVES AND USES THEREOF 6,444,708 B2 9/2002 Rudolph et al. 6,765,008 B1 7, 2004 Chen (75) Inventors: Jonathan Laird Gross, Cranbury, NJ 6,967.201 B1 1 1/2005 Briner et al. (US); Gary Paul Stack, Ambler, PA 7,045,545 B1 5, 2006 Briner et al. (US); Dahui Zhou, East Brunswick, NJ 2002/0183395 Al 12/2002 Argentieri et al. (US); Hong Gao, Belle Mead, NJ (US) 2004.0029949 A1 2/2004 Argentieri et al. 2004/0235856 Al 11/2004 McMurray et al. (73) Assignee: Wyeth, Madison, NJ (US) 2005, 0124692 A1 6/2005 Gross et al. 2005.01434.52 A1 6/2005 Gross et al. (*) Notice: Subject to any disclaimer, the term of this 2005/0261347 A1 11/2005 Gross et al. patent is extended or adjusted under 35 2006,008.9405 A1 4, 2006 Zhou U.S.C. 154(b) by 99 days. 2006/0111438 A1 5/2006 Gontcharov et al. 2006/0241172 A1 10, 2006 Zhou et al. (21) Appl. No.: 11/408,319 2006/0241176 A1 10, 2006 Stack et al. 2006/0246551 A1 11/2006 Stack et al. (22) Filed: Apr. 21, 2006 2006/0247276 A1 11/2006 Gross et al. 2006/0252825 A1 11/2006 Tadayon et al. (65) Prior Publication Data 2006/0258639 A1 11/2006 Logue et al. 2006/0258711 A1 11/2006 Rosenzweig-Lipson US 2006/O24727.6 A1 Nov. -

Clinical Efficacy and Tolerability of Cimicoxib in Dogs with Osteoarthritis: a Multicentre Prospective Study
Open Journal of Veterinary Medicine, 2014, 4, 78-90 Published Online May 2014 in SciRes. http://www.scirp.org/journal/ojvm http://dx.doi.org/10.4236/ojvm.2014.45010 Clinical Efficacy and Tolerability of Cimicoxib in Dogs with Osteoarthritis: A Multicentre Prospective Study Joanna Murrell1*, Erik Grandemange2, Frédérique Woehrle2, Julie Menard3, Kate White4 1School of Veterinary Sciences, University of Bristol, Bristol, UK 2Vétoquinol S.A., Centre de Recherche, Lure, France 3Vétoquinol S.A., Paris, France 4School of Veterinary Medicine and Science, University of Nottingham, Nottingham, UK Email: *[email protected] Received 10 March 2014; revised 10 April 2014; accepted 25 April 2014 Copyright © 2014 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Background: Cimicoxib is a coxib recently licensed in Europe for pain and inflammation associated with osteoarthritis (OA), and the management of perioperative pain due to orthopaedic or soft tissue surgery. Purpose: This prospective study was to complete the product information for the end users by providing additional scientific data obtained after a thirty-day treatment course of cimicoxib in dogs with OA, and to collect owners’ feedback. Data were collected from nine Euro- pean countries with 492 client owned dogs recruited to the trial. Dogs were treated once daily with 2 mg/kg cimicoxib orally. Immediately before, at Day (D) 15 and D30 after the start of treat- ment veterinarians and owners scored body condition, appetite, locomotion, lameness, pain on palpation and manipulation of the joint and joint effusion (veterinarians) and dog demeanor and well being (owners).