BMJ Open Is Committed to Open Peer Review. As Part of This Commitment We Make the Peer Review History of Every Article We Publish Publicly Available
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
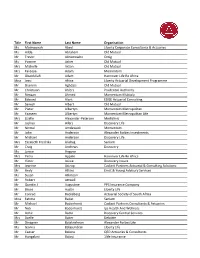
2020 Convention Attendee List 201007.Xlsx
Title First Name Last Name Organisation Ms Maimoonah Abed Liberty Corporate Consultants & Actuaries Ms Hilda Abraham Old Mutual Mr Trevor Abromowitz Omig Ms Yvonne Acker Old Mutual Mrs Michelle Acton Old Mutual Mrs Fareeya Adam Momentum Mr Obaidullah Adam Hannover Life Re Africa Miss Jessi Africa Liberty Actuarial Development Programme Mr Shamim Aghdasi Old Mutual Mr Christiaan Ahlers Prudential Authority Mr Rimaan Ahmed Momentum Multiply Mr Edward Alant EDGE Actuarial Consulting Mr Sanesh Albert Old Mutual Mr Pieter Albertyn Momentum Metropolitan Mr Yaaseen Albertyn Momentum Metropolitan Life Mrs Lizelle Alexander‐Petersen Mediclinic Mr Joshua Allers Discovery Life Mr Nirmal Amdawadi Momentum Mr John Anderson Alexander Forbes Investments Mr Michael Anderson Discovery Life Mrs Elizabeth Fredrika Andrag Sanlam Mr Craig Andrews Discovery Ms Janice Angove Mrs Petro Appelo Hannover Life Re Africa Mr Victor Asiwe Discovery Insure Mrs Jeanine Astrup Cadiant Partners Actuarial & Consulting Solutions Mr Andy Atkins Ernst & Young Advisory Services Ms Susan Atkinson Mr Robert Attwell Mr Quintin J Augustine PPS Insurance Company Mr Bruce Austin Liberty Life Mr Conrad Backeberg Actuarial Society of South Africa Miss Fatima Badat Sanlam Mr Michael Badenhorst Cadiant Partners Consultants & Actuaries Mr Nick Badenhorst Ips Health And Wellness Mr Adhir Badul Discovery Central Services Mrs Azelle Baker Deloitte Mr Dinagren Balakrishnan Alexander Forbes Life Ms Gavina Balasundran Liberty Life Mr Caesar Balona QED Actuaries & Consultants Mr Hungalani Baloyi -

Spring 2018 Dean's List
Spring 2018 Dean's List Approximately 9,104 Iowa State University students have been recognized for outstanding academic achievement by being named to the Spring Semester 2018 Dean's List. Students named to the Dean's List must have earned a grade point average of at least 3.50 on on a 4.00 scale while carrying a minimum of 12 credit hours of graded course work. NAME CURRICULUM YR CITY STATE COUNTRY Afina Syaurah A Aziz Chemical Engineering 3 Alexandra Lea Aaberg Biology 4 Coralville, IA Pauline E. Aamodt Bioinformatics and Computational Biology 4 Woodbury, MN Aayushi Management Information Systems 4 Charles Alex Abate Management 4 Saint Charles, IL Drew Matthew Abbas Agricultural Studies 4 Alexander, IA Omar M. Abbas Computer Engineering 4 Chicago, IL Brianne Elizabeth Abbasi Animal Science 4 Cedar Rapids, IA Emily Elaine Abbasi Animal Science 4 Cedar Rapids, IA Anthony Michael Abbate Materials Engineering 4 Cary, IL Leah Sophie Abbott Agriculture Specials 5 Paige Jeanette Abbott Veterinary Medicine 4 Elgin, IL Josiah Michael Abbott Industrial Technology 3 West Des Moines, IA Ibrahim Abdalla Marketing 4 West Des Moines, IA Mohamed S. Abdennadher Chemical Engineering 3 Uzma Liyana Abdul Razak Supply Chain Management 4 Kaitlyn Abdulghani Biology 4 Johnston, IA Eric Thomas Abens Industrial Technology 2 Manson, IA Alex‐Marie E. Ablan Graphic Design 4 Eagan, MN Steven Joseph Abramsky Industrial Design 4 Cuba City, WI John Matthew Aceto Landscape Architecture 4 Urbandale, IA Cody Acevedo Animal Ecology 3 Gilbert, IA Melissa Marie Achenbach English 2 Underwood, IA Keely Sarah Acheson Agricultural Business 4 Rushville, IL Ross Allen Ackerman Political Science 3 Harrisburg, SD Aaron Benjamin Ackerman Liberal Arts and Sciences Specials (Non‐Degree) 5 Ames, IA Lexi Ann Ackerman Event Management 4 Rock Rapids, IA Georgia Kate Ackley Food Science (AGLS) 2 Fredericksburg, IA Karly Jo Ackley Kinesiology and Health 2 Manvel, ND Ashley Brianne Acree Sociology 4 Savanna, IL Alexis M. -

Including Ardrossan Beaumont-Nisku
Calgary Reception Archive Copy Please Return Yellow Pages ii Area Code 403 : SHERWOOD PARK INCLUDING ARDROSSAN 3! BEAUMONT-NISKU r ui and Area m ( &• ,1 .^nr... aft :y; •- iv V- 'f('; •• ..f -.'•v-r. ^ ' V.'' /} I.I • r •••••, •- •^•-L''•"'T--.?* ''i -a': V • •• ii: •*';.. < •I, -A'. ' •; -1 -• ' '' . '•• I, ' I '•> ' • l' I '• •' .' • •• ' - •; -'''V'f ••••'• r . •••••. .:.'X\.vv:' v.-.. • •--•.••.•.•a- ''.V .' f DINOSAUR PROVINCIAL PARK NEAR BROOKS \DC7 We bring your world to you. Recyclable SHERWOOD PARK See also Ardrossan •— Fort Saskatchewan for other customers located In this area. For detailed AGT listings, see Index Page at front of directory SERVICE CALLS REPAIR SERVICE BUSINESS OFFICE (Before calling, please see Repair Guide, (8 AM - 4:30 PM Mon. - Frl.) 493-4400 blue-edged page 11) GENERAL INQUIRIES BURIED CABLE LOCATION (Main Swltctil>oard) 493-3110 (Alberta let Call) PHONE CENTRE Sherwood Park Mall, 2020 Sherwood Drive 464-2500 MERGENCY CALLS SHERWOOD PARK FIRE FIRE— POLICE— AMBULANCE DEAF, HEARING & SPEECH AMBULANCE IMPAIRED EMERGENCY 467-2344 9-1-1 POISON CENTRE (Toll-free) 1-800-332-1414 POLICE-RCMP 467-7741 If Busy Call Calgary 1-670-1414 LOCAL CALLING AREA Alberta Beach — Ardrossan — Beaumont — Bon Accord — Bruderheim — Calmer — Chlpman — Devon — Edmonton — Edmonton International Airport — Fort Saskatchewan — Gibbons — Hay Lakes — Keephills — Lamont — Leduc — Legal — Millet — Morlnville — Namao — New Sarepta — Nisku — Onoway — Redwater — St Albert — St Michael — Spruce Grove — Stony Plain — Thorsby — Tofield — Wabamun Just dial the 7-digit number TELEPHONE ACCOUNTS MAY BE PAID AT: Chartered Banks, Credit Unions, Treasury Branches and Authorized Agents AEC PIPELINES A DIV OF A&L HEATING & A Abcan Moving & Storage ALBERTA ENERGY CO LTD AGTLIMITED VENTILATION . -

Wetenschappelijk Jaarverslag UZA - Publicaties 2019 Opgesteld Door Danny Mathysen I.O.V
Wetenschappelijk Jaarverslag UZA - Publicaties 2019 Opgesteld door Danny Mathysen i.o.v. Prof. dr. Guy Hans (Medisch Directeur) 1. Aboukais, R; Verbraeken, B; Leclerc, X; Gautier, C; Henon, H; Vermandel, M; Menovsky, T; Lejeune, JP Superficial temporal artery-middle cerebral artery anastomosis patency correlates with cerebrovascular reserve in adult moyamoya syndrome patients NEUROCHIRURGIE 65 (4): 146-151 WOS:000484880500002 Article 2. Aboukais, R; Verbraeken, B; Leclerc, X; Gautier, C; Vermandel, M; Bricout, N; Lejeune, JP; Menovsky, T Protective STA-MCA bypass to prevent brain ischemia during high-flow bypass surgery: case series of 10 patients ACTA NEUROCHIRURGICA 161 (6): 1207-1214 WOS:000468224800023 Article 3. Abrahams, AC; Dendooven, A; van Der Veer, JW; Wientjes, R; Toorop, RJ; Bleys, RLAW; Hendrickx, APA; van Leeuwen, MS; de Lussanet, QG; Verhaar, MC; Stapper, G; Nguyen, TQ DIRECT COMPARISON OF THE THICKNESS OF THE PARIETAL PERITONEUM USING PERITONEAL BIOPSY AND ULTRASONOGRAPHY OF THE ABDOMINAL WALL IN PATIENTS TREATED WITH PERITONEAL DIALYSIS PERITONEAL DIALYSIS INTERNATIONAL 39 (5): 455-464 WOS:000484823800008 Article 4. Aernouts, T; De Boeck, M; Vleugels, J; Peeters, M; De Bruyne, G A Cryotherapeutic Device for Preventing Nail Toxicity During Chemotherapy: Comparison of Three Cooling Strategies ADVANCES IN HUMAN FACTORS AND ERGONOMICS IN HEALTHCARE AND MEDICAL DEVICES 779 (): 30-36 WOS:000471292600004 Proceedings Paper Wetenschappelijk Jaarverslag UZA - Publicaties 2019 Opgesteld door Danny Mathysen i.o.v. Prof. dr. Guy Hans (Medisch Directeur) 5. Aerts, O; Bracke, A; Goossens, A; Meuleman, V; Lambert, J Titanium dioxide nanoparticles and frontal fibrosing alopecia: cause or consequence? JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY 33 (1): E45-E46 WOS:000456213500034 Letter 6. -

Resource Review
April 2021 Resource Review The REFINE Resource Review is a collection of materials to keep you updated on research related to food assistance products and malnutrition. Resources identified and added between January and March 2021 are detailed below and are available on the REFINE website. The goal of Research Engagement on Food Innovations for Nutritional Effectiveness (REFINE) is to enhance the accessibility to, and exchange of, rigorous, operational and policy relevant research on nutrition-directed interventions that improve nutrition in both emergency and non- emergency contexts. REFINE is a product of the Food Aid Quality Review (FAQR) project, which is funded by the United States Agency for International Development’s Bureau for Humanitarian Assistance (USAID/BHA) and provides actionable recommendations on ways to improve nutrition among vulnerable populations for whom the direct distribution of food assistance can make a significant impact. Feel free to direct any questions, comments, and additional resources you come across to Natalie Volin, the REFINE Research Assistant, at [email protected] or at [email protected]. Ongoing Clinical Trials This section includes ongoing trials on five clinical trial registries searched by REFINE. No clinical trials identified in this issue. Published Food Assistance Product Studies This section includes publications from clinical trials testing food assistance products and peer-reviewed evidence, including reports and evaluations from programs using these products. On REFINE, these resources are tagged according to country, nutritional problem studied, intervention used, study type, year, and author. de Kok, B., Moore, K., Jones, L., Vanslambrouck, K., Toe, L.C., Ouédraogo, M., Ganaba, R., de Pee, S., Bedford, J., Lachat, C. -

Re-Imagining Love and Intimacy in the Poetry of Gabeba Baderoon, Ingrid De Kok, and Makhosazana Xaba
Re-Imagining Love and Intimacy in the Poetry of Gabeba Baderoon, Ingrid De Kok, and Makhosazana Xaba Jenny Bozena Du Preez Submitted in fulfilment of the requirements for the degree Magister Artium in the Faculty of Arts at the Nelson Mandela Metropolitan University January 2014 Supervisor: Professor Mary West Contents i. Acknowledgements ii. Abstract I. Introduction 1 1. Chapter One: Gabeba Baderoon – „Silences, Secrets, Fragments‟ 1.1. Introduction 10 1.2. Avoiding Sex as Spectacle in ―The Dream in the Next Body‖ 12 1.3. The Sensual in ―Cinnamon‖ 17 1.4. Traces of Intimacy in ―Finding You‖ 21 1.5. Acknowledging the Ex-Lover in ―Old photographs‖ 25 1.6. Reading Absence in ―Today she is not here‖ 29 1.7. Moments in Marriage: ―The night before we married‖ and ―Primal scene‖ 33 1.8. Conclusion 38 2. Chapter Two: Ingrid de Kok – „The Delicious Fiction, Love‟ 2.1. Introduction 39 2.2. Re-Thinking ‗Lack‘ in ―Woman in the glass‖ 41 2.3. Sex in the Sillier Body in ―To a would-be lover‖ 47 2.4. Critiquing Clichéd ―Words of love‖ 51 2.5. Re-Imagining Photographic Representation in ―Woman, leaning away‖ 55 2.6. Writing the Aging Body in ―After forty‖ 60 2.7. Re-Writing the ―Aubade‖ 64 2.8. An Impression of Intimacy in ―Brush stroke‖ 68 2.9. Conclusion 72 3. Chapter Three: Makhosazana Xaba - „Revolution in Poetic Language‟ 3.1. Introduction 74 3.2. The Stark Reality of Rape in ―The silence of a lifetime‖ 76 3.3. Reclaiming the Gaze in ―Your eyes‖ 82 3.4. -

Mycotoxins in Feed and Food Chain • Filippo Rossi Mycotoxins in Feed and Food Chain
Mycotoxins in Feed and Food Chain • Filippo Rossi Mycotoxins in Feed and Food Chain Present Status and Future Concerns Edited by Filippo Rossi Printed Edition of the Special Issue Published in Toxins www.mdpi.com/journal/toxins Mycotoxins in Feed and Food Chain Mycotoxins in Feed and Food Chain Present Status and Future Concerns Editor Filippo Rossi MDPI • Basel • Beijing • Wuhan • Barcelona • Belgrade • Manchester • Tokyo • Cluj • Tianjin Editor Filippo Rossi Catholic University Italy Editorial Office MDPI St. Alban-Anlage 66 4052 Basel, Switzerland This is a reprint of articles from the Special Issue published online in the open access journal Toxins (ISSN 2072-6651) (available at: https://www.mdpi.com/journal/toxins/special issues/mycotoxins feed food chain). For citation purposes, cite each article independently as indicated on the article page online and as indicated below: LastName, A.A.; LastName, B.B.; LastName, C.C. Article Title. Journal Name Year, Article Number, Page Range. ISBN 978-3-03936-874-7 (Hbk) ISBN 978-3-03936-875-4 (PDF) c 2020 by the authors. Articles in this book are Open Access and distributed under the Creative Commons Attribution (CC BY) license, which allows users to download, copy and build upon published articles, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. The book as a whole is distributed by MDPI under the terms and conditions of the Creative Commons license CC BY-NC-ND. Contents About the Editor .............................................. vii Filippo Rossi A Long Road to Safer Food Reprinted from: Toxins 2020, 12, 453, doi:10.3390/toxins12070453 ................. -

The Family of Hendrick Cock of Amsterdam
THE NEW YORK GENEALOGICAL AND BIOGRAPHICAL RECORD VOLUME 142 NUMBER 2 APRIL 2011 Family of Joshua4 Stephens/Stevens and Christiana Dutcher of Connecticut, Pennsylvania, Massachusetts, and New York Editorial Note: /R\DO6XEMHFWVRU´8QIDLWKIXOO3HUMXUHG 3HUVRQVµ" Persons in Suffolk County, Long Island, Who Took the Oath of Allegiance and Peaceable Behavior, 1778 Family of Hendrick Cock of Amsterdam Streeter Immigrants of Greene and Steuben Counties: Elizabeth (Streeter) Faulkner, Thomas Streeter, and William Streeter (concluded ) Gertrude Barber, Minnie Cowen, and Ray Sawyer: The Sisters Who Indexed New York (continued ) Romer Family of Westchester County (concluded ) The New York Genealogical and Biographical Society Officers W. FRANCIS PRICE, JR., Chairman ROBERT G. GOELET, Vice Chairman MCKELDEN SMITH, President and Trustee ex Officio LUKE IVES PONTIFELL, Secretary ROBERT F. HENDRICKSON, Treasurer Board of Trustees Term Expiring March 2012 Term Expiring March 2013 Term Expiring March 2014 R. BRANDON FRADD DEBRA TANNER ABELL, MD ELBRUN KIMMELMAN JOHN C. HARVEY ELIZABETH L. BRADLEY, Ph.D. LUKE IVES PONTIFELL ROBERT F. HENDRICKSON ROBERT G. GOELET ROBERT S. ROBERSON ANITA A. LUSTENBERGER, CG, FGBS HENRY B. ROBERTS, FGBS W. FRANCIS PRICE, JR. M. DAVID SHERRILL JEANNE SLOANE WADDELL W. STILLMAN Trustees Emeriti TIMOTHY F. BEARD, FASG, FGBS HENRY C. B. LINDH, FGBS HENRY B. HOFF, CG, FASG, FGBS WALTER WILMERDING, FGBS WILLIAM P. JOHNS E. LISK WYCKOFF, JR. Fellows of the New York Genealogical and Biographical Society TIMOTHY F. BEARD, FASG HARRY MACY, JR., FASG LESLIE CORN, CG HENRY B. ROBERTS CHARLOTTE MEGILL HIX MERIWETHER C. SCHMID HENRY B. HOFF, CG, FASG EDWIN L. SIBERT ROGER D. JOSLYN, CG, FASG WALTER WILMERDING HENRY C.B. -

1 Mile Results
Race Name: The Midmar Mile Race Date: February 11-12 2017 Location: Durban, South Africa Distance: 1 Mile Rank Name Country Gender Div Age Group Time Points Entrants 1 Matthew Meyer South Africa M N 19&U 0:18:13 100.00 10912 2 Chad Ho South Africa M N 20-29 0:18:18 99.99 3 Chip Peterson USA M N 20-29 0:18:20 99.99 4 Ferry Weertman Netherlands M N 20-29 0:18:26 99.98 5 Tyrone Kruger South Africa M N 20-29 0:18:55 99.97 7 Neil Fair South Africa M N 20-29 0:18:59 99.97 8 Luke Erwee South Africa M N 20-29 0:19:19 99.96 9 Ashley Twichell USA F N 20-29 0:19:24 99.95 10 Holly Hibbott United Kingdom F N 20-29 0:19:37 99.95 11 Myles Brown South Africa M N 20-29 0:19:37 99.95 13 Martin Binedell South Africa M N 20-29 0:19:48 99.94 14 Chad Michau South Africa M N 20-29 0:19:51 99.93 15 Brendon Levy South Africa M N 20-29 0:19:55 99.93 16 Azyan Makhija South Africa M N 20-29 0:20:00 99.92 17 Dante Nortje South Africa M N 20-29 0:20:02 99.92 18 Calvin Stott South Africa M N 20-29 0:20:13 99.91 19 Luca Holtzhausen South Africa M N 19&U 0:20:16 99.90 20 Connor Grobler South Africa M N 20-29 0:20:21 99.89 22 Hanre Van Geffen South Africa M N 20-29 0:20:23 99.89 23 Evan Fair South Africa M N 20-29 0:20:23 99.89 24 Eric Le Roux South Africa M N 20-29 0:20:26 99.88 26 Ruben Du Pisanie South Africa M N 20-29 0:20:30 99.87 27 Gary Albertyn South Africa M N 40-49 0:20:30 99.86 28 Harrison Coulter South Africa M N 20-29 0:20:33 99.86 30 Robyn Kinghorn South Africa F N 20-29 0:20:34 99.85 38 Terry Heller South Africa M N 40-49 0:20:35 99.84 39 Cameron Pennell -

Effect of Balanced Energy-Protein Supplementation During Pregnancy and Lactation on Birth Outcomes and Infant Growth in Rural Bu
BMJ Open: first published as 10.1136/bmjopen-2020-038393 on 24 March 2021. Downloaded from PEER REVIEW HISTORY BMJ Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form (http://bmjopen.bmj.com/site/about/resources/checklist.pdf) and are provided with free text boxes to elaborate on their assessment. These free text comments are reproduced below. ARTICLE DETAILS TITLE (PROVISIONAL) THE EFFECT OF BALANCED ENERGY-PROTEIN SUPPLEMENTATION DURING PREGNANCY AND LACTATION ON BIRTH OUTCOMES AND INFANT GROWTH IN RURAL BURKINA FASO : STUDY PROTOCOL FOR A RANDOMIZED CONTROLLED TRIAL AUTHORS Vanslambrouck, Katrien; de Kok, Brenda; Toe, Laeticia Celine; De Cock, Nathalie; Ouedraogo, Moctar; Dailey-Chwalibog, Trenton; Hanley Cook, Giles; Ganaba, Rasmané; Lachat, Carl; Huybregts, Lieven; Kolsteren, Patrick VERSION 1 – REVIEW REVIEWER Megan Bourassa New York Academy of Sciences, USA REVIEW RETURNED 08-Apr-2020 GENERAL COMMENTS This study protocol is well written and well thought out. The introduction provides some global and LMIC-specific interventions, but given that many nutrition interventions are context-specific, it would be nice to include some information on the previous studies http://bmjopen.bmj.com/ done in Burkina Faso. There have been a number of recent studies on nutrient interventions during pregnancy in Burkina Faso, including multiple micronutrient supplements and lipid-based supplements. In this overview, I would also incorporate how the MISAME III study fits into the previous MISAME projects. REVIEWER Leila Nikniaz Tabriz health services management research center. Tabriz. on September 26, 2021 by guest. Protected copyright. University of Medical Sciences, Tabriz, Iran REVIEW RETURNED 25-Jul-2020 GENERAL COMMENTS I think this is a complete protocol for evaluating the effect of the supplement on birth outcomes. -

MARCH 30, 2010 CREATIVE SERVICES-10436 CREATIVE Research at the University of Alberta
This event is sponsored by the Office of the Vice-President (Research) The CelebraTion of researCh & innovaTion is CoordinaTed by The Office of the Vice-President (Research) 203 Telus Centre University of Alberta special thanks to staff in the following areas for their help in planning this event: > The Office of the President > The Office of the Provost and Vice-President (Academic) > The Office of the Vice-President (External Relations) > The Office of the Vice-President (Research) > The Office of the Registrar and Student Awards > Faculty of Graduate Studies and Research > Postdoctoral Fellows Office > Campus Security accessibility services provided by Specialized Support and Disability Services MARCH 30, 2010 CREATIVE SERVICES-10436 CREATIVE Research at the University of Alberta This event celebrates the breadth of research and creative activity at our University and the impact of our contributions to Albertan and Canadian society as well as around the world. Our goal is to support the talented people at the U of A—faculty, support staff, and students—with the resources and services to create one of the world’s great universities for the public good. The University of Alberta is committed to building on its strengths in research and the creative arts by partnering in innovative ways with government, industry, and others to develop a knowledge- based economy and to enhance interdisciplinary links with our community and beyond. Funding from federal and provincial agencies, foundations, and private sponsors is critical to the success of the University of Alberta. We gratefully acknowledge these organizations’ continuing support of the research and creative life of the University. -
Protein Supplementation During Pregnancy and Lactation on Birth Outcomes and Infant Growth in Rural Burkina Faso: Study Protocol for a Randomised Controlled Trial
Open access Protocol BMJ Open: first published as 10.1136/bmjopen-2020-038393 on 24 March 2021. Downloaded from Effect of balanced energy- protein supplementation during pregnancy and lactation on birth outcomes and infant growth in rural Burkina Faso: study protocol for a randomised controlled trial Katrien Vanslambrouck ,1 Brenda de Kok ,1 Laeticia Celine Toe ,1,2 Nathalie De Cock ,1 Moctar Ouedraogo ,3 Trenton Dailey- Chwalibóg ,1 Giles Hanley- Cook ,1 Rasmané Ganaba ,3 Carl Lachat ,1 Lieven Huybregts ,4 Patrick Kolsteren 1 To cite: Vanslambrouck K, de ABSTRACT Strengths and limitations of this study Kok B, Toe LC, et al. Effect Introduction Adequate nutrition during pregnancy is of balanced energy- protein crucial to both mother and child. Maternal malnutrition can ► This trial will help to fill the evidence gap on the ef- supplementation during be the cause of stillbirth or lead to poor birth outcomes pregnancy and lactation on fect of balanced energy- protein (BEP) supplements such as preterm delivery and small-for -gesta tional- birth outcomes and infant in pregnant and lactating women on birth outcomes age newborns. There is a probable positive effect of growth in rural Burkina Faso: and infant growth. providing pregnant women a balanced energy- protein study protocol for a randomised ► Formative research to select the most suitable BEP (BEP) food supplement, but more evidence is needed. The controlled trial. BMJ Open supplement ensured that the selected BEP is well MIcronutriments pour la SAnté de la Mère et de l’Enfant 2021;11:e038393. doi:10.1136/ accepted by the study population.