Report on Biodiversity Value of GBWLS (Final Draft) 2017
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Amphibian Abundance and Detection Trends During a Large Flood in a Semi-Arid Floodplain Wetland
Herpetological Conservation and Biology 11:408–425. Submitted: 26 January 2016; Accepted: 2 September 2016; Published: 16 December 2016. Amphibian Abundance and Detection Trends During a Large Flood in a Semi-Arid Floodplain Wetland Joanne F. Ocock1,4, Richard T. Kingsford1, Trent D. Penman2, and Jodi J.L. Rowley1,3 1Centre for Ecosystem Science, School of Biological, Earth and Environmental Sciences, UNSW Australia, Sydney, New South Wales, 2052, Australia 2Centre for Environmental Risk Management of Bushfires, Institute of Conservation Biology and Environmental Management, University of Wollongong, Wollongong, New South Wales 2522, Australia 3Australian Museum Research Institute, Australian Museum, 6 College St, Sydney, New South Wales 2010, Australia 4Corresponding author, email: [email protected] Abstract.—Amphibian abundance and occupancy are often reduced in regulated river systems near dams, but com- paratively little is known about how they are affected on floodplain wetlands downstream or the effects of actively managed flows. We assessed frog diversity in the Macquarie Marshes, a semi-arid floodplain wetland of conserva- tion significance, identifying environmental variables that might explain abundances and detection of species. We collected relative abundance data of 15 amphibian species at 30 sites over four months, coinciding with a large natural flood. We observed an average of 39.9 ± (SE) 4.3 (range, 0-246) individuals per site survey, over 47 survey nights. Three non-burrowing, ground-dwelling species were most abundant at temporarily flooded sites with low- growing aquatic vegetation (e.g., Limnodynastes tasmaniensis, Limnodynastes fletcheri, Crinia parinsignifera). Most arboreal species (e.g., Litoria caerulea) were more abundant in wooded habitat, regardless of water permanency. -

Vedapatasaalas in Andhra Pradesh
Vedapatasaalas in Andhra Pradesh Dr. K. Varalakshmi Deputy Director, Sanskri Academy, Osmania University, Hyderabad Andhra Pradesh 1. Sri Sita Rama VedaSamskrutha VidyaPeethamu Charitable Trust. Regn.No.:25/01-02/dit (E). Jagadevapur, Medak Disrict, Andhra Pradesh, India Pin : 502281 Phone : 91-40-27538908 or 91-9989699311 Email : admin [at] vedabhoomi.org Email does not work Krishna Yajurvedam and rest belong to Krishna Yajurveda Smartham Telugu and Sanskrit Publications Sanskrit Vyakaranam and Sanskrit Kavyas Audio downlodable CDs of AudioTextBooks at http://www.vedabhoomi.org/SanskritChanting.html Sri Adi Sankara's Bhasyopeta of Isavasya, Katha and Taittiriya Upanishad, Bhattoji Dikshita's Siddhanta Kaumudi and Sanskrit MahaKavyas like Megha Sandesham, Kumara Sambhavam, Kiratarjuneeyam and Raghu Vamsham Support students and Pathashala so such audio renderings can be provided free of cost Scrollable photo gallery http://www.vedabhoomi.org Veda Patashaala Visi by Ani and Divya 2.Hari Hara Veda Vidya Peetham Sri Satyanarayana Swamy Devasthanam VEDA (Vedic Education and Devotional Academy) Hari Hara Veda Vidya Peetham (Vedic Educational Society) Regn. No. 7064/2001 H. No. 6-146, Sreenagar 3rd Line, Kothagudem . 507 101 Khammam District Andhra Pradesh, India Ph. No: +1-91-8744-243640 A branch in Milpitas, California, US Audio files site http://siliconvalleytemple.net 3.Vedabhavan, Secunderabad Sankara Bhaktha Sabha Trust (Regd) VEDA BHAVAN, 58 and 59 Road no 1, Chandragiri Colony ( west) Neredmet Secunderabad- 500 058 Tel nos 040- 2722 7669 and 2722 9775 Email ghanapati [at] gmail.com Website http://www.vedabhavan.org (under construction) The number of students in the veda Patashala are approx 100. Video. 4.Sarvaraya Educational Trust, Kapileswarpuram Zamindra.s House Gandhinagar, Kakinada-533004, Andhra Pradesh (Supported by MSRVVP) Video clip Information on Vedapathashala sponsored by Sri Shirdi Sai Baba Temple in North America in 1991. -

Supplementary Information
Supplementary Information Space-time variability of ambient PM2.5 diurnal pattern over India from 18-years (2000-2017) of MERRA-2 reanalysis data 1Kunal Bali*, 1,2Sagnik Dey, 1Dilip Ganguly and 3, 4Kirk R. Smith 1Centre for Atmospheric Sciences, Indian Institute of Technology Delhi, Hauz Khas, New Delhi, India 2Centre of Excellence for Research on Clean Air, Indian Institute of Technology Delhi, Hauz Khas, New Delhi, India 3School of Public Health, University of California, Berkeley, USA 4Collaborative Clean Air Policy Center, New Delhi, India *Corresponding author: [email protected] The supplementary information contains twenty figures and one table. Figure R1. Diurnal climatology and correlation (hourly average of 80 sites) of PM2.5 exposure the period of 2009-2017 over the Indian region. Figure S1. Hourly climatology of PM2.5 exposure during January-February (JF) for the period of 2000-2017 over the Indian region. Figure S2. Hourly climatology of PM2.5 exposure during March-April-May (MAM) for the period of 2000-2017 over the Indian region. Figure S3. Hourly climatology of PM2.5 exposure during June-July-August- Septemebr (JJAS) for the period of 2000-2017 over the Indian region. Figure S4. Hourly climatology of PM2.5 exposure during October-November- December (OND) for the period of 2000-2017 over the Indian region. Figure S5. Hourly anomaly of PM2.5 exposure during January-February (JF) for the period of 2000-2017 over the Indian region. Figure S6. Hourly anomaly of PM2.5 exposure during March-April-May (MAM) for the period of 2000-2017 over the Indian region. -

ARAZPA YOTF Infopack.Pdf
ARAZPA 2008 Year of the Frog Campaign Information pack ARAZPA 2008 Year of the Frog Campaign Printing: The ARAZPA 2008 Year of the Frog Campaign pack was generously supported by Madman Printing Phone: +61 3 9244 0100 Email: [email protected] Front cover design: Patrick Crawley, www.creepycrawleycartoons.com Mobile: 0401 316 827 Email: [email protected] Front cover photo: Pseudophryne pengilleyi, Northern Corroboree Frog. Photo courtesy of Lydia Fucsko. Printed on 100% recycled stock 2 ARAZPA 2008 Year of the Frog Campaign Contents Foreword.........................................................................................................................................5 Foreword part II ………………………………………………………………………………………… ...6 Introduction.....................................................................................................................................9 Section 1: Why A Campaign?....................................................................................................11 The Connection Between Man and Nature........................................................................11 Man’s Effect on Nature ......................................................................................................11 Frogs Matter ......................................................................................................................11 The Problem ......................................................................................................................12 The Reason -
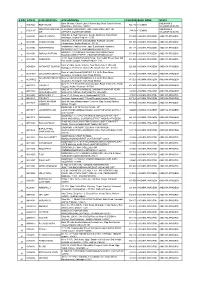
S No Atm Id Atm Location Atm Address Pincode Bank
S NO ATM ID ATM LOCATION ATM ADDRESS PINCODE BANK ZONE STATE Bank Of India, Church Lane, Phoenix Bay, Near Carmel School, ANDAMAN & ACE9022 PORT BLAIR 744 101 CHENNAI 1 Ward No.6, Port Blair - 744101 NICOBAR ISLANDS DOLYGUNJ,PORTBL ATR ROAD, PHARGOAN, DOLYGUNJ POST,OPP TO ANDAMAN & CCE8137 744103 CHENNAI 2 AIR AIRPORT, SOUTH ANDAMAN NICOBAR ISLANDS Shop No :2, Near Sai Xerox, Beside Medinova, Rajiv Road, AAX8001 ANANTHAPURA 515 001 ANDHRA PRADESH ANDHRA PRADESH 3 Anathapur, Andhra Pradesh - 5155 Shop No 2, Ammanna Setty Building, Kothavur Junction, ACV8001 CHODAVARAM 531 036 ANDHRA PRADESH ANDHRA PRADESH 4 Chodavaram, Andhra Pradesh - 53136 kiranashop 5 road junction ,opp. Sudarshana mandiram, ACV8002 NARSIPATNAM 531 116 ANDHRA PRADESH ANDHRA PRADESH 5 Narsipatnam 531116 visakhapatnam (dist)-531116 DO.NO 11-183,GOPALA PATNAM, MAIN ROAD NEAR ACV8003 GOPALA PATNAM 530 047 ANDHRA PRADESH ANDHRA PRADESH 6 NOOKALAMMA TEMPLE, VISAKHAPATNAM-530047 4-493, Near Bharat Petroliam Pump, Koti Reddy Street, Near Old ACY8001 CUDDAPPA 516 001 ANDHRA PRADESH ANDHRA PRADESH 7 Bus stand Cudappa, Andhra Pradesh- 5161 Bank of India, Guntur Branch, Door No.5-25-521, Main Rd, AGN9001 KOTHAPET GUNTUR 522 001 ANDHRA PRADESH ANDHRA PRADESH Kothapeta, P.B.No.66, Guntur (P), Dist.Guntur, AP - 522001. 8 Bank of India Branch,DOOR NO. 9-8-64,Sri Ram Nivas, AGW8001 GAJUWAKA BRANCH 530 026 ANDHRA PRADESH ANDHRA PRADESH 9 Gajuwaka, Anakapalle Main Road-530026 GAJUWAKA BRANCH Bank of India Branch,DOOR NO. 9-8-64,Sri Ram Nivas, AGW9002 530 026 ANDHRA PRADESH ANDHRA PRADESH -

List of Officers Who Attended Courses at NCRB
List of officers who attened courses at NCRB Sr.No State/Organisation Name Rank YEAR 2000 SQL & RDBMS (INGRES) From 03/04/2000 to 20/04/2000 1 Andhra Pradesh Shri P. GOPALAKRISHNAMURTHY SI 2 Andhra Pradesh Shri P. MURALI KRISHNA INSPECTOR 3 Assam Shri AMULYA KUMAR DEKA SI 4 Delhi Shri SANDEEP KUMAR ASI 5 Gujarat Shri KALPESH DHIRAJLAL BHATT PWSI 6 Gujarat Shri SHRIDHAR NATVARRAO THAKARE PWSI 7 Jammu & Kashmir Shri TAHIR AHMED SI 8 Jammu & Kashmir Shri VIJAY KUMAR SI 9 Maharashtra Shri ABHIMAN SARKAR HEAD CONSTABLE 10 Maharashtra Shri MODAK YASHWANT MOHANIRAJ INSPECTOR 11 Mizoram Shri C. LALCHHUANKIMA ASI 12 Mizoram Shri F. RAMNGHAKLIANA ASI 13 Mizoram Shri MS. LALNUNTHARI HMAR ASI 14 Mizoram Shri R. ROTLUANGA ASI 15 Punjab Shri GURDEV SINGH INSPECTOR 16 Punjab Shri SUKHCHAIN SINGH SI 17 Tamil Nadu Shri JERALD ALEXANDER SI 18 Tamil Nadu Shri S. CHARLES SI 19 Tamil Nadu Shri SMT. C. KALAVATHEY INSPECTOR 20 Uttar Pradesh Shri INDU BHUSHAN NAUTIYAL SI 21 Uttar Pradesh Shri OM PRAKASH ARYA INSPECTOR 22 West Bengal Shri PARTHA PRATIM GUHA ASI 23 West Bengal Shri PURNA CHANDRA DUTTA ASI PC OPERATION & OFFICE AUTOMATION From 01/05/2000 to 12/05/2000 1 Andhra Pradesh Shri LALSAHEB BANDANAPUDI DY.SP 2 Andhra Pradesh Shri V. RUDRA KUMAR DY.SP 3 Border Security Force Shri ASHOK ARJUN PATIL DY.COMDT. 4 Border Security Force Shri DANIEL ADHIKARI DY.COMDT. 5 Border Security Force Shri DR. VINAYA BHARATI CMO 6 CISF Shri JISHNU PRASANNA MUKHERJEE ASST.COMDT. 7 CISF Shri K.K. SHARMA ASST.COMDT. -

Andhra Pradesh Tourism Development
ANDHRA PRADESH TOURISM DEVELOPMENT CORPORATION LTD # D.NO.55-17-2 to 4, Fifth Floor, Industrial Estate, Stalin Corporate Building Auto Nagar, Vijayawada -520 007, Website: www.tourism.ap.gov.in Phone: 0866-2552969 Fax: 0866-2552964, Email: [email protected] INVITATION FOR EXPRESSION OF INTEREST FOR OPERATING TOUR PACKAGES FOR TIRUMALA & OTHER PLACES UNDER JOINT PARTNERSHIP MODE ------------------------------------------------------------------------------------------------------------------------------------------ BASIC TERMS AND CONDITIONS FOR APPROVAL FOR DRAFTING THE EOI DOCUMENT PART-A 1. BACKGROUND A. Andhra Pradesh Tourism Development Corporation Limited, (APTDC), a Government of Andhra Pradesh fully owned company, intends to enlarge its tourism promotional activities, inter-alia, its tour packages in association with the private bus operators for Tirumala and other places of tourist importance. B. Under this Scheme of Joint Partnership Mode (JPM) while the APTDC provides the Back End Services (BES) at cost, the private Bus Operator (Service Provider), will operate the buses as per the standards, specifications, guidelines and conditions stipulated by APTDC including fixing of the ticket price by APTDC. 2. PACKAGE TOURS A. Back End Services and Tariff i) Following are the Back End Services (BES) that will be provided by APTDC as a Part of the Package Tour. a) Fresh up/ Accommodation of Tourists at Package Tour Destination. b) Breakfast/ Lunch/Snacks /Dinner. c) Guide services. d) Darshan /Boating/ Rope Way/ Sound & Light show and Entry Tickets wherever applicable. ii) The cost of back end services with reference to each individual component involved in the tour will be fixed by APTDC from time to time. The tariff for Package Tour includes cost of BES. -

Water Quality Monitoring on Tirumala and Tirupati, Andhra Pradesh, India
Available online a t www.derpharmachemica.com Scholars Research Library Der Pharma Chemica, 2012, 4 (3):1074-1079 (http://derpharmachemica.com/archive.html) ISSN 0975-413X CODEN (USA): PCHHAX Water Quality monitoring on Tirumala and Tirupati, Andhra Pradesh, India K. Raju and T. Damodharam* Dept. of Environmental Sciences, SVU College of Sciences, S.V.University, Tirupati-517502 , A.P. India. ______________________________________________________________________________ ABSTRACT An attempt has been made to evaluate the water quality of supplemented and ground water in Tirumala and Tirupati, Chittoor District, Andhra Pradesh, India. The Tirumala and Tirupati are the most popular pilligramage and education areas in Andhra Pradesh. Twelve areas of Tirumala and Tirupati have been selected, where the peoples are used supplemented and groundwater for drinking purpose, and the water samples were subjected to systematic analysis with a view to understand the potability of drinking water sources. The values obtained for different parameters have been compared with the standard values given by ISI/ICMR/ WHO and the variations were notable for the parameters like electrical conductivity, total dissolved solids, total hardness and nitrates for few samples. Medical survey has been carried out to study the harmful effects on the society due to these four parameters at the areas - Tiruchanur, Renigunta and Karakambadi. Key words: Physico-chemical parameters; Total dissolved solids; Total hardness; Electrical conductivity. ______________________________________________________________________________ INTRODUCTION Water is one of the most essential components for the existence of life on earth. Although water pollution is an age- old problem, in this modern age, the problems like growing population, sewage disposal, industrial waste, radioactive waste, etc. have polluted our water resources so much that about 75 % rivers and streams, not only of India but also of all the countries, contain polluted waters [1]. -

List of PD Consumer Under MIDC Area Having Arrears
DISTRICTWISE LIST OF PERMANENT DISCONNECTED CONSUMERS IN MIDC AREA CONSUMER SANCTIONED CONNECTED INT NAME OF DISTRICT CONSUMER NAME ADDRESS LINE 1 ADDRESS LINE 2 ADDRESS LINE 3 TARIFF CATEGORY PR ARREARS Total Arrears NUMBER LOAD LOAD ARREARS AKOLA 313970008509 M/S SHREE GAJANAN INDUSTRIES PLOT NO R 1 MIDC BALAPUR KASTAKHED / BHIKUNDKHED TQ DIST AKOLA LT INDUSTRIAL UPTO 27 HP 15 15 10374.1 19.3 10393.5 AKOLA 322210008397 PUSPASHRI DALL MILL C/O. SANTOSH S. PLOT NO. A 14 MIDC MURTIZAPUR. LT INDUSTRIAL ABOVE 27 HP 90 90 100.0 0.0 100.0 AKOLA 322210152056 SHEKHAR APPARAO WAKODE SAFFRON INDUSTRIES PLOT NO M.I.D.C MURTIZAPUR TQ MURT LT INDUSTRIAL ABOVE 27 HP 30 30 32521.9 3546.8 36068.7 AKOLA 322210152072 KARAN FEBRICATION PROP. SHRI. RAMESH PLOT NO.B 16 MIDC MURTIZAPUR U 1 CEN LT INDUSTRIAL UPTO 27 HP 15 15 34007.5 3913.5 37921.1 AKOLA 322210152081 M/S ADARSH IND SHRI.VILAS GENDUJI KH MURTIZAPUR MIDC TQ MURTIZAPUR LT INDUSTRIAL ABOVE 27 HP 70 70 354472.3 15132.0 369604.3 AKOLA 310219045040 ATC TELECOM INFRASTRUCTURE PVT.LTD. PLOT NO.57MIDC PHASE III AKOLA LT INDUSTRIAL UPTO 27 HP 15 15 714.9 34.2 749.1 AKOLA 310210773397 M/S ELECTICAL SPARE CORPORATION M I D C AKOLA LT INDUSTRIAL UPTO 27 HP 20 20 3249.1 0.0 3249.1 AKOLA 310210820719 M/S L M TRIVEDI AND CO M I D C PHASE I AKOLA LT INDUSTRIAL UPTO 27 HP 3 3 2935.0 658.5 3593.5 AKOLA 310210837158 M/S SAKAS FEEDS PROP V S KOTNIS MIDC III SHIVAR AKOLA LT INDUSTRIAL UPTO 27 HP 2 2 3328.8 457.8 3786.5 AKOLA 310210754686 SHRI S Y PATIL PRAKASH INDUSTRIES SHIVANI SHIVAR M I D C AKOLA LT INDUSTRIAL -

Announcement
21/11/2020 Announcement RECRUITMENT OF CLERKS IN INDIAN BANK IBPS CLERKS CRP IX: CONDUCT OF PRE EMPLOYMENT VERIFICATION PROCESS ******** Reference is invited to provisional allotment of candidates for the post of Clerk in Indian Bank through IBPS CLERKS CRP IX. The selected candidates are advised to report at the venue on the date mentioned against their name for completion of pre employment verification process as per list appended. Intimation letter is emailed to the candidates advising them to bring originals / copies of all the requisite documents mentioned therein. Only those candidates who are found eligible in the pre employment verification process will be issued Offer of Appointment for joining shortly. in the Bank’s services Candidates are advised to make necessary arrangement well in advance and report at the venue. Assistant General Manager (HRM) Venue for verification: Reg No Name State Applied Date for reporting Indian Bank Zonal Office 1800145019 A GRACIA ESTHER TAMIL NADU MADURAI 25.11.2020 1800019709 A NANDHINI TAMIL NADU SALEM 25.11.2020 1800255037 A SAHAYA JOE AUGUSTINE TAMIL NADU KARAIKUDI 27.11.2020 1800419578 A T RAJEE TAMIL NADU TIRUNELVELI 27.11.2020 1800708188 AAKANSHA SRIVASTAVA CHHATTISGARH RAIPUR 25.11.2020 1800463176 AAKARSHITA DELHI DELHI (CENTRAL) 25.11.2020 1800563056 AAKASH POKHRIYAL DELHI DELHI (CENTRAL) 25.11.2020 1800152971 AARTHI ALEXANDER TAMIL NADU CUDDALORE 25.11.2020 1800109321 AASTHA PUNJAB LUDHIANA 25.11.2020 1800207087 AASTHA GUPTA RAJASTHAN JAIPUR 25.11.2020 1800198375 AAYAN KISHORE KHOUND -

Bevezetés És Célkitűzések 2
AZ IHARKÚTI KÉSŐ-KRÉTA KÉTÉLTŰ FAUNA VIZSGÁLATA TAXONÓMIAI, FUNKCIONÁLIS ANATÓMIAI, PALEOÖKOLÓGIAI ÉS PALEOBIOGEOGRÁFIAI SZEMPONTBÓL SZENTESI ZOLTÁN FÖLDTUDOMÁNYI DOKTORI ISKOLA Dr. Gábris Gyula egyetemi tanár, PhD FÖLDTAN – GEOFIZIKA PROGRAM Prof. Dr. Mindszenty Andrea, PhD Témavezető: Dr. Görög Ágnes, egyetemi docens, PhD Konzulensek: Dr. Venczel Márton, tudományos főkutató, PhD Dr. Ősi Attila, kutatócsoport vezető, PhD EÖTVÖS LORÁND TUDOMÁNYEGYETEM TERMÉSZETTUDOMÁNYI KAR ŐSLÉNYTANI TANSZÉK Tartalomjegyzék Bevezetés és célkitűzések 2. Az iharkúti felső-kréta gerinces lelőhely földrajzi elhelyezkedése és földtani háttere 4. A magyarországi mezozoós gerinces lelőhelyek kutatástörténete 11. A modern kétéltűek (Lissamphibia) kutatásának rövid története 12. A vizsgált anyag és munkamódszerek 20. Az iharkúti kétéltű leletek leírása és összehasonlítása 37. A Bakonybatrachus és a Hungarobatrachus izomzatának és mozgásmódjának rekonstrukciója 71. A leletek értelmezése paleobiológiai szempontból 95. Az iharkúti lelőhelyről előkerült kétéltű csontok tafonómiai jellemzői, és a leletek értelmezése paleoökológiai szempontból 97. A leletek értelmezése paleobiogeográfiai szempontból 102. Összefoglalás 113. Abstract 114. Köszönetnyilvánítás 115. Hivatkozott irodalom 116. 2 Bevezetés és célkitűzések A mikrogerinces lelőhelyek fontos forrásai a paleontológiai információknak, ezért fontos a vizsgált rétegek üledékeinek leiszapolása és az iszapolási maradék gondos átvizsgálása. Nagymennyiségű fosszília nyerhető általa, mely sokkal több információt nyújthat -

Mechanisms Underlying Inhibition of Muscle Disuse Atrophy During Aestivation in the Green-Striped Burrowing Frog, Cyclorana Alboguttata
Mechanisms underlying inhibition of muscle disuse atrophy during aestivation in the green-striped burrowing frog, Cyclorana alboguttata Beau Daniel Reilly Bachelor of Marine Studies (Hons.) A thesis submitted for the degree of Doctor of Philosophy at The University of Queensland in 2014 School of Biological Sciences Abstract In most mammals, extended inactivity or immobilisation of skeletal muscle (e.g. bed- rest, limb-casting or hindlimb unloading) results in muscle disuse atrophy, a process which is characterised by the loss of skeletal muscle mass and function. In stark contrast, animals that experience natural bouts of prolonged muscle inactivity, such as hibernating mammals and aestivating frogs, consistently exhibit limited or no change in either skeletal muscle size or contractile performance. While many of the factors regulating skeletal muscle mass are known, little information exists as to what mechanisms protect against muscle atrophy in some species. Green-striped burrowing frogs (Cyclorana alboguttata) survive in arid environments by burrowing underground and entering into a deep, prolonged metabolic depression known as aestivation. Throughout aestivation, C. alboguttata is immobilised within a cast-like cocoon of shed skin and ceases feeding and moving. Remarkably, these frogs exhibit very little muscle atrophy despite extended disuse and fasting. The overall aim of the current research study was to gain a better understanding of the physiological, cellular and molecular basis underlying resistance to muscle disuse atrophy in C. alboguttata. The first aim of this study was to develop a genomic resource for C. alboguttata by sequencing and functionally characterising its skeletal muscle transcriptome, and to conduct gene expression profiling to identify transcriptional pathways associated with metabolic depression and maintenance of muscle function in aestivating burrowing frogs.