CHAPTER 11 Gases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tutorial 2 FORMULAS, PERCENTAGE COMPOSITION
T-6 Tutorial 2 FORMULAS, PERCENTAGE COMPOSITION, AND THE MOLE FORMULAS: A chemical formula shows the elemental composition of a substance: the chemical symbols show what elements are present and the numerical subscripts show how many atoms of each element there are in a formula unit. Examples: NaCl: one sodium atom, one chlorine atom in a formula unit CaCl2: one calcium atom, two chlorine atoms in a formula unit Mg3N2: three magnesium atoms, two nitrogen atoms in a formula unit The presence of a metal in a chemical formula indicates an ionic compound, which is composed of positive ions (cations) and negative ions (anions). A formula with only nonmetals indicates a + molecular compound (unless it is an ammonium, NH4 , compound). Only ionic compounds are considered in this Tutorial. There are tables of common ions in your lecture text, p 56 (cations) and p 57 (anions). A combined table of these same ions can be found on the inside back cover of the lecture text. A similar list is on the next page; all formulas needed in this and subsequent Tutorial problems can be written with ions from this list. Writing formulas for ionic compounds is very straightforward: TOTAL POSITIVE CHARGES MUST BE THE SAME AS TOTAL NEGATIVE CHARGES. The formula must be neutral. The positive ion is written first in the formula and the name of the compound is the two ion names. EXAMPLE: Write the formula for potassium chloride. The name tells you there are potassium, K+, and chloride, Cl–, ions. Each potassium ion is +1 and each chloride ion is -1: one of each is needed, and the formula for potassium chloride is KCl. -

Avogadro Number, Boltzmann Constant, Planck Constant, Information Theory, Comparative and Relative Uncertainties
American Journal of Computational and Applied Mathematics 2018, 8(5): 93-102 DOI: 10.5923/j.ajcam.20180805.02 h, k, NA: Evaluating the Relative Uncertainty of Measurement Boris Menin Mechanical & Refrigeration Consultation Expert, Beer-Sheba, Israel Abstract For verification of the measurement accuracy of fundamental physical constants, the CODATA unique technique is used. This procedure is a complex process based on a careful discussion of the input data, and the justification and construction of tables of values sufficient for the direct use of the relative uncertainty are conducted using modern advanced statistical methods and powerful computers. However, at every stage of data processing, researchers must rely on common sense, that is, an expert conclusion. In this article, the author proposes a theoretical and informational grounded justification for calculating the relative uncertainty by using the comparative uncertainty. A detailed description of the data and the processing procedures do not require considerable time. Examples of measurements results of three fundamental constants are analysed, applying information-oriented approach. Comparison of the achieved relative and comparative uncertainties is introduced and discussed. Keywords Avogadro number, Boltzmann constant, Planck constant, Information theory, Comparative and relative uncertainties theories of physics can be proved by carefully studying the 1. Introduction numerical values of these constants, determined from different experiments in different fields of physics. It is expected that in 2018 that the International System of One needs to note the main feature of the CODATA Units (SI) will be redefined on the basis of certain values of technique in determining the relative uncertainty of one some fundamental constants [1]. -

X-Ray Crystal Density Method to Determine the Avogadro and Planck Constants
X-ray Crystal Density Method to Determine the Avogadro and Planck Constants Horst Bettin Physikalisch-Technische Bundesanstalt Braunschweig, Germany Proposed New Definition of the Kilogram The kilogram, symbol kg, is the SI unit of mass. It is defined by taking the fixed numerical value of the Planck constant h to be 6.626 070 040 x 10 -34 when expressed in the unit J s, which is equal to kg m2 s-1, where the metre and the second c ∆ν are defined in terms of and Cs . *) X represents one or more digits to be added at the time the new definition is finally adopted. α 2 - = M (e )c NAh 2 R∞ N h −10 A = 3.990 312 7110(18) × 10 Js/mol, Amedeo Avogadro Max Planck with relative uncertainty of 0.45 × 10 -9 (1776-1856) (1858-1947) Page 2 of 19 Avogadro Constant Definition of Avogadro constant NA • Number of molecules per mol • 6.022... x 10 23 mol -1 Amedeo Avogadro Current definition of mol (1776-1856) • Number “entities” like 12 C atoms in 12 g • i. e. 6.022... x 10 23 12 C atoms have a mass of 12 g 12 12 g/mol = NA m( C ) Faraday constant F = NA e (e: elementary charge) Molar gas constant R = NA k (k: Boltzmann constant) Page 3 of 19 Counting Atoms: XRCD Method 3 Use of a silicon crystal! 1. Volume a0 of the unit cell 3 2. Volume of an atom: a0 /8 3. Volume V of a sphere 4. Number N of the atoms 8 V8 VM N = = ⋅ mol A N 3 3 a0 a0msphere Page 4 of 19 Lattice parameter measurement (INRIM) d220 (2011)=192014712.67(67) am d220 (2014)=192014711.98(34) am -9 ur(2014) = 1.8 x 10 Page 5 of 19 Lattice parameter set-up at PTB S M1 M M2 AL AA OH Page 6 of 19 Sphere Interferometer of PTB mK-temperature stabilisation Camera 1 Camera 2 Fizeau- Fizeau- Collimator Objective 1 Objective 2 Diode laser Page 7 of 19 Diameter results (2014) Relative Mean apparent Sphere Lab. -

2•Stoichiometry: Chemical Arithmetic Formula Conventions (1 Of
Superscripts used to show the charges on ions Mg2+ the 2 means a 2+ charge (lost 2 electrons) Subscripts 2•Stoichiometry: Chemical Arithmetic used to show numbers of atoms in a formula unit Formula Conventions H2SO4 two H’s, one S, and 4 O’s (1 of 24) Coefficients used to show the number of formula units 2Br– the 2 means two individual bromide ions Hydrates CuSO4 • 5 H2O some compounds have water molecules included stoichiometry study of the quantitative relationships in chemical formulas and equations. atomic mass weighted average mass of an atom, found on the periodic table 2•Stoichiometry: Chemical Arithmetic formula mass sum of the atomic masses of the Stoichiometry Terms atoms in a formula molecular mass sum of the atomic masses of the (2 of 24) atoms in a molecular formula gram molecular mass molecular mass written in grams molar mass same as gram molecular mass empirical formula formula reduced to lowest terms Formula or molecular mass is found by simply summing the atomic masses (on the periodic table) of each atom in a formula. H2SO4 2•Stoichiometry: Chemical Arithmetic 1.01 + 1.01 + 32.06 + 16.0 + 16.0 + 16.0 + 16.0 = 98.08 u Calculating Formula Mass 2(1.01) + 32.06 + 4(16.0) = 98.06 u or 98.06 g/mole (3 of 24) Generally, round off your answers to the hundredths or tenths place. Don’t round off too much (98.06 g/mol or 98.1 g/mol is OK, but don’t round off to 98 g/mol) Units Use u or amu if you are referring to one atom or molecule A mole (abbreviated mol) is a certain number of things. -

Solute Concentration: Molality
5/25/2012 Colligative Properties of Solutions . Colligative Properties: • Solution properties that depend on concentration of solute particles, not the identity of particles. Previous example: vapor pressure lowering. Consequences: change in b.p. and f.p. of solution. © 2012 by W. W. Norton & Company Solute Concentration: Molality . Changes in boiling point/freezing point of solutions depends on molality: moles of solute m kg of solvent • Preferred concentration unit for properties involving temperature changes because it is independent of temperature. © 2012 by W. W. Norton & Company 1 5/25/2012 Calculating Molality Starting with: a) Mass of solute and solvent. b) Mass of solute/ volume of solvent. c) Volume of solution. © 2012 by W. W. Norton & Company Sample Exercise 11.8 How many grams of Na2SO4 should be added to 275 mL of water to prepare a 0.750 m solution of Na2SO4? Assume the density of water is 1.000 g/mL. © 2012 by W. W. Norton & Company 2 5/25/2012 Boiling-Point Elevation and Freezing-Point Depression . Boiling Point Elevation (ΔTb): • ΔTb = Kb∙m • Kb = boiling point elevation constant of solvent; m = molality. Freezing Point Depression (ΔTf): • ΔTf = Kf∙m • Kf = freezing-point depression constant; m = molality. © 2012 by W. W. Norton & Company Sample Exercise 11.9 What is the boiling point of seawater if the concentration of ions in seawater is 1.15 m? © 2012 by W. W. Norton & Company 3 5/25/2012 Sample Exercise 11.10 What is the freezing point of radiator fluid prepared by mixing 1.00 L of ethylene glycol (HOCH2CH2OH, density 1.114 g/mL) with 1.00 L of water (density 1.000 g/mL)? The freezing-point-depression constant of water, Kf, is 1.86°C/m. -

2 Weighing and Preparation of Aqueous Solutions
2 WEIGHING AND PREPARATION OF AQUEOUS SOLUTIONS Theory In chemistry, a solution is a homogeneous mixture composed of two or more substances. In such a mixture, a solute is dissolved in another substance, known as a solvent. An aqueous solution is a solution in which the solvent is water. Concentration is the measure of how much of a given substance (solute) there is mixed with another substance (solvent). This can apply to any sort of chemical mixture, but most frequently the concept is limited to homogeneous solutions, where it refers to the amount of solute in a solution. For scientific or technical applications, concentration is expressed in a quantitative way. There are a number of different ways to quantitatively express concentration; the most common are listed below. They are based on mass or volume or both. Depending on what they are based on it is not always trivial to convert one measure to the other, because knowledge of the density might be needed to do so. At times this information may not be available, particularly if the temperature varies. Mass can be determined at a precision of ~ 0.1 mg on a routine basis with an analytical balance. Both solids and liquids are easily quantified by weighing. Some units of concentration - particularly the most popular one (molarity) - require to express the mass of substance in the moles. The mol is the SI basic unit that measures an amount of substance. „One mole contains exactly 6.022 140 76 × 1023 elementary entities. This number is the −1 fixed numerical value of the Avogadro constant, NA, when expressed in the unit mol and is called the Avogadro number.“ The previous definition was based on the number of carbon atoms in 12 g of material and was the following; a mol is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram (or 12 g) of carbon-12 (12C), where the carbon – 12 atoms are unbound, at rest and in their ground state. -

Topic720 Composition: Mole Fraction: Molality: Concentration a Solution Comprises at Least Two Different Chemical Substances
Topic720 Composition: Mole Fraction: Molality: Concentration A solution comprises at least two different chemical substances where at least one substance is in vast molar excess. The term ‘solution’ is used to describe both solids and liquids. Nevertheless the term ‘solution’ in the absence of the word ‘solid’ refers to a liquid. Chemists are particularly expert at identifying the number and chemical formulae of chemical substances present in a given closed system. Here we explore how the chemical composition of a given system is expressed. We consider a simple system prepared using water()l and urea(s) at ambient temperature and pressure. We designate water as chemical substance 1 and urea as chemical substance j, so that the closed system contains an aqueous solution. The amounts of the two substances are given by n1 = = ()wM11 and nj ()wMjj where w1 and wj are masses; M1 and Mj are the molar masses of the two chemical substances. In these terms, n1 and nj are extensive variables. = ⋅ + ⋅ Mass of solution, w n1 M1 n j M j (a) = ⋅ Mass of solvent, w1 n1 M1 (b) = -1 For water, M1 0.018 kg mol . However in reviewing the properties of solutions, chemists prefer intensive composition variables. Mole Fraction The mole fractions of the two substances x1 and xj are given by the following two equations: =+ =+ xnnn111()j xnnnjj()1 j (c) += Here x1 x j 10. In general terms for a system comprising i - chemical substances, the mole fraction of substance k is given by equation (d). ji= = xnkk/ ∑ n j (d) j=1 ji= = Hence ∑ x j 10. -

16.4 Calculations Involving Colligative Properties 16.4
chem_TE_ch16.fm Page 491 Tuesday, April 18, 2006 11:27 AM 16.4 Calculations Involving Colligative Properties 16.4 1 FOCUS Connecting to Your World Cooking instructions for a wide Guide for Reading variety of foods, from dried pasta to packaged beans to frozen fruits to Objectives fresh vegetables, often call for the addition of a small amount of salt to the Key Concepts • What are two ways of 16.4.1 Solve problems related to the cooking water. Most people like the flavor of expressing the concentration food cooked with salt. But adding salt can of a solution? molality and mole fraction of a have another effect on the cooking pro- • How are freezing-point solution cess. Recall that dissolved salt elevates depression and boiling-point elevation related to molality? 16.4.2 Describe how freezing-point the boiling point of water. Suppose you Vocabulary depression and boiling-point added a teaspoon of salt to two liters of molality (m) elevation are related to water. A teaspoon of salt has a mass of mole fraction molality. about 20 g. Would the resulting boiling molal freezing-point depression K point increase be enough to shorten constant ( f) the time required for cooking? In this molal boiling-point elevation Guide to Reading constant (K ) section, you will learn how to calculate the b amount the boiling point of the cooking Reading Strategy Build Vocabulary L2 water would rise. Before you read, make a list of the vocabulary terms above. As you Graphic Organizers Use a chart to read, write the symbols or formu- las that apply to each term and organize the definitions and the math- Molality and Mole Fraction describe the symbols or formulas ematical formulas associated with each using words. -

Avogadro's Constant
Avogadro's Constant Nancy Eisenmenger Question Artem, 8th grade How \Avogadro constant" was invented and how scientists calculated it for the first time? Answer What is Avogadro's Constant? • Avogadros constant, NA: the number of particles in a mole • mole: The number of Carbon-12 atoms in 0.012 kg of Carbon-12 23 −1 • NA = 6:02214129(27) × 10 mol How was Avogadro's Constant Invented? Avogadro's constant was invented because scientists were learning about measuring matter and wanted a way to relate the microscopic to the macroscopic (e.g. how many particles (atoms, molecules, etc.) were in a sample of matter). The first scientists to see a need for Avogadro's constant were studying gases and how they behave under different temperatures and pressures. Amedeo Avogadro did not invent the constant, but he did state that all gases with the same volume, pressure, and temperature contained the same number of gas particles, which turned out to be very important to the development of our understanding of the principles of chemistry and physics. The constant was first calculated by Johann Josef Loschmidt, a German scientist, in 1865. He actually calculated the Loschmidt number, a constant that measures the same thing as Avogadro's number, but in different units (ideal gas particles per cubic meter at 0◦C and 1 atm). When converted to the same units, his number was off by about a factor of 10 from Avogadro's number. That may sound like a lot, but given the tools he had to work with for both theory and experiment and the magnitude of the number (∼ 1023), that is an impressively close estimate. -

2.1 Relative Atomic and Molecular Masses, the Avogadro Constant, and the Mole Calculations AQA Chemistry
2.1 Relative atomic and molecular AQA Chemistry masses, the Avogadro constant, and the mole Calculations The Avogadro constant Specification reference 3.1.2.2 MS 0.1 Recognise and use expressions in decimal and ordinary form MS 0.4 Use calculators to find and use power, exponential, and logarithmic functions MS 1.1Use an appropriate number of significant figures. Learning objectives After completing the worksheet you should be able to: carry out calculations using the Avogadro constant carry out calculations using numbers in standard and ordinary form substitute numerical values into algebraic equations, and change the subject of equations report calculations to an appropriate number of significant figures. Introduction One mole of any substance contains the same number of particles as the number of atoms in 12 g of carbon-12. This number of particles is a huge number and is called the Avogadro constant. It has a value of 6.02 1023 and is given the units of mol−1 (per mole). The relative atomic mass of an element in grams contains 6.02 1023 atoms or one mole of that element. The relative formula mass of a compound in grams contains 6.02 1023 particles (molecules or ions) or one mole of that compound. Therefore we use the term molar mass to describe the relative atomic or formula mass of a substance in grams and give it the unit g mol−1. To calculate the number of particles in a certain mass of a substance we can use the following equation. mass m (g) Number of particles = ´ 6.02 ´ 1023(mol-1) molar mass M (g mol-1) Worked example Question 1 How many molecules are there in 42.5 g of ammonia, NH3? Answer © Oxford University Press 2015 www.oxfordsecondary.co.uk/acknowledgements This resource sheet may have been changed from the original 1 2.1 Relative atomic and molecular AQA Chemistry masses, the Avogadro constant, and the mole Calculations Step 1 Calculate the molar mass of ammonia using the relative atomic masses of nitrogen and hydrogen from the periodic table. -

Zumdahl Chapter 5 Gases
5 Gases Contents 5.1 Pressure • Units of Pressure 5.2 The Gas Laws of Boyle, Charles, and Avogadro • Boyle’s Law • Charles’s Law • Avogadro’s Law 5.3 The Ideal Gas Law 5.4 Gas Stoichiometry • Molar Mass of a Gas 5.5 Dalton’s Law of Partial Pressures • Collecting a Gas over Water 5.6 The Kinetic Molecular Theory of Gases • Pressure and Volume (Boyle’s Law) • Pressure and Temperature • Volume and Temperature (Charles’s Law) • Volume and Number of Moles (Avogadro’s Law) • Mixture of Gases (Dalton’s Law) • Deriving the Ideal Gas Law • The Meaning of Temperature • Root Mean Square Velocity 5.7 Effusion and Diffusion • Effusion • Diffusion 5.8 Real Gases 5.9 Characteristics of Several Real Gases 5.10 Chemistry in the Atmosphere The steaming fumaroles located in Bjarnarflag, Iceland release a variety of gases. 178 Matter exists in three distinct physical states: gas, liquid, and solid. Although rel- atively few substances exist in the gaseous state under typical conditions, gases are very important. For example, we live immersed in a gaseous solution. The earth’s atmosphere is a mixture of gases that consists mainly of elemental nitrogen (N2) and oxygen (O2). The atmosphere both supports life and acts as a waste receptacle for the exhaust gases that accompany many industrial processes. The chemical reactions of these waste gases in the atmosphere lead to various types of pollution, including smog and acid rain. The gases in the atmosphere also shield us from harmful radiation from the sun and keep the earth warm by reflecting heat radiation back toward the earth. -
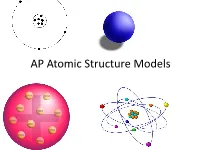
AP Atomic Structure Models What Is a Model?
AP Atomic Structure Models What is a Model? On a scrap piece of paper, write down your definition of a model with at least two examples. A model is a representation of an object, idea, action, or concept. Models A model in science is usually an approximation of the “real thing.” This means that all models have simplifications that may lead to omitted information. Models When a model is selected for a specific purpose, it should be able to help predict a “behavior.” The Four Historical Models of the Atom 1. Particle model 2. Plum Pudding model 3. Rutherford model 4. Bohr model Modern Model Quantum Mechanical Model Why do we have so many historical models? Are some of them still useful? When we initially began the process of determining the structure of the atom, we first had to overcome the prevailing scientific concept that matter was continuous. This idea of continuous matter was from Aristotle. (a Greek philosopher 384-322 B.C.E.) Particle Model John Dalton’s four ideas about the particle nature of matter helped push forward the acceptance of the first model. 1. All elements are composed of tiny indivisible particles called atoms. 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. John Dalton 3. Atoms of different elements can physically mix together or can chemically combine in simple whole-number ratios to form compounds. 4. Chemical reactions occur when atoms are separated, joined, or rearranged. Atoms of one element, however, are never changed into atoms of another element as a result of a chemical reaction.