Local Impacts of White-Nose Syndrome on the Foraging Ecology of Insectivorous Bats
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
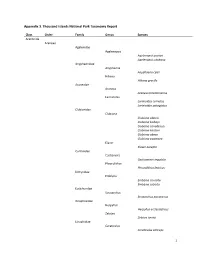
1 Appendix 3. Thousand Islands National Park Taxonomy Report
Appendix 3. Thousand Islands National Park Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis potteri Agelenopsis utahana Anyphaenidae Anyphaena Anyphaena celer Hibana Hibana gracilis Araneidae Araneus Araneus bicentenarius Larinioides Larinioides cornutus Larinioides patagiatus Clubionidae Clubiona Clubiona abboti Clubiona bishopi Clubiona canadensis Clubiona kastoni Clubiona obesa Clubiona pygmaea Elaver Elaver excepta Corinnidae Castianeira Castianeira cingulata Phrurolithus Phrurolithus festivus Dictynidae Emblyna Emblyna cruciata Emblyna sublata Eutichuridae Strotarchus Strotarchus piscatorius Gnaphosidae Herpyllus Herpyllus ecclesiasticus Zelotes Zelotes hentzi Linyphiidae Ceraticelus Ceraticelus atriceps 1 Collinsia Collinsia plumosa Erigone Erigone atra Hypselistes Hypselistes florens Microlinyphia Microlinyphia mandibulata Neriene Neriene radiata Soulgas Soulgas corticarius Spirembolus Lycosidae Pardosa Pardosa milvina Pardosa moesta Piratula Piratula canadensis Mimetidae Mimetus Mimetus notius Philodromidae Philodromus Philodromus peninsulanus Philodromus rufus vibrans Philodromus validus Philodromus vulgaris Thanatus Thanatus striatus Phrurolithidae Phrurotimpus Phrurotimpus borealis Pisauridae Dolomedes Dolomedes tenebrosus Dolomedes triton Pisaurina Pisaurina mira Salticidae Eris Eris militaris Hentzia Hentzia mitrata Naphrys Naphrys pulex Pelegrina Pelegrina proterva Tetragnathidae Tetragnatha 2 Tetragnatha caudata Tetragnatha shoshone Tetragnatha straminea Tetragnatha viridis -

LITERA TURE CITED CLOKEY, IW 951. Flora Of
24 JOURNAL OF THE LEPIDOPI'ERISTS' SOCIETY LITERA TURE CITED CLOKEY, I. W. 951. Flora of the Charleston Mountains, Clark County, Nevada. Univ. Calif. Publ. Botany, No. 24. EMMEL, J. F. & O. SHIELDS. The biology of Plebejus (Icaricia) shasta in the western United Sta s (Lycaenidae). J. Res. Lepid., in press. FERRIS, C. D. 976. Revisionary notes on Plebejus (Icaricia) shasta (Edwards). Bull. Allyn Mus m, No. 36. GARTH,]. S. 1 8. Report of the Lorquin Entomological Society of Los Angeles. Proc. So. Calif. A ad. Sci. 27: 93-94. HALL, E. R. 1946. Mammals of Nevada. Univ. Calif. Press, Berkeley. HOWE, W. H. 1975. The butterflies of North America. Doubleday, Garden City, N.Y. JOHNSON, N. K. 1965. The breeding avifaunas of the Sheep and Spring ranges in southern Nevada. Condor 67: 93-124. SMITHE, F. B. 1975. Naturalist's color guide. Am. Mus. Nat. Hist., New York. Journal of the Lepidopterists' Societ!J 34(1), 1980,24 THE IDENTITY OF THE PLANT REFERRED TO AS ANDROMEDA BY W. T. M. FORBES The name Andromeda has been used ambiguously in the lepidopteran literature. A striking example is in its mention as a larval host for Datana major Grote and Robinson and Datana ranaeceps (Guerin) by Forbes (1948, Lepidoptera of New York ... II: Cornell U. Agric. Expt. Sta. Mem. 274, p. 215). The ranges of both of these species lie almost entirely to the south and east of the only species of the genus Andromeda covered by Fernald (1950, Gray's Manual of Botany, 8th ed. Amer. Book Co., N.Y., p. -

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

Bioblitz! OK 2019 - Cherokee County Moth List
BioBlitz! OK 2019 - Cherokee County Moth List Sort Family Species 00366 Tineidae Acrolophus mortipennella 00372 Tineidae Acrolophus plumifrontella Eastern Grass Tubeworm Moth 00373 Tineidae Acrolophus popeanella 00383 Tineidae Acrolophus texanella 00457 Psychidae Thyridopteryx ephemeraeformis Evergreen Bagworm Moth 01011 Oecophoridae Antaeotricha schlaegeri Schlaeger's Fruitworm 01014 Oecophoridae Antaeotricha leucillana 02047 Gelechiidae Keiferia lycopersicella Tomato Pinworm 02204 Gelechiidae Fascista cercerisella 02301.2 Gelechiidae Dichomeris isa 02401 Yponomeutidae Atteva aurea 02401 Yponomeutidae Atteva aurea Ailanthus Webworm Moth 02583 Sesiidae Synanthedon exitiosa 02691 Cossidae Fania nanus 02694 Cossidae Prionoxystus macmurtrei Little Carpenterworm Moth 02837 Tortricidae Olethreutes astrologana The Astrologer 03172 Tortricidae Epiblema strenuana 03202 Tortricidae Epiblema otiosana 03494 Tortricidae Cydia latiferreanus Filbert Worm 03573 Tortricidae Decodes basiplaganus 03632 Tortricidae Choristoneura fractittana 03635 Tortricidae Choristoneura rosaceana Oblique-banded Leafroller moth 03688 Tortricidae Clepsis peritana 03695 Tortricidae Sparganothis sulfureana Sparganothis Fruitworm Moth 03732 Tortricidae Platynota flavedana 03768.99 Tortricidae Cochylis ringsi 04639 Zygaenidae Pyromorpha dimidiata Orange-patched Smoky Moth 04644 Megalopygidae Lagoa crispata Black Waved Flannel Moth 04647 Megalopygidae Megalopyge opercularis 04665 Limacodidae Lithacodes fasciola 04677 Limacodidae Phobetron pithecium Hag Moth 04691 Limacodidae -

Field Observations of Divergent Resting Behavior Among Hickory Feeding Catocala Larvae (Noctuidae)
Journal of the Lepidopterists' SOciety 36(4), 1982,303 GENERAL NOTES FIELD OBSERVATIONS OF DIVERGENT RESTING BEHAVIOR AMONG HICKORY FEEDING CATOCALA LARVAE (NOCTUIDAE) Catoeala are known for their sympatric diversity, with at least 35 species present at almost any forested locality in southern New England (see Sargent, 1976, Legion of night: The underwing moths, Univ. Massachusetts Press, Amherst, MA; also personal observation). Of these, about one third feed on Juglandaceae (hickories and walnuts). The precise foodplant preferences of these Juglandaceae feeders will be the topic of another paper (Schweitzer, in prep.) and are also being investigated by L. F. Gall. Sargent (1976, op. cit.; 1977, J. Lepid. Soc. 31:1-16) suggested that food may not be an important limiting factor in Catocala evolution. "Competition" for predator avoidance might be more important in Catocala ecology. Such competition might be manifest in different larval resting behaviors, which might serve to inhibit effective search image formation by predators, especially birds. Sargent (1976, op. cit.) reports laboratory obser vations of instar specific resting behaviors in Catocala dejecta Strecker and C. retecta Grote which were reared in plastic containers on Carya ovata. Although similar in ap pearance the two species grow at very different rates and so avoid having similar size and behavior simultaneously. Little is known about field resting habits of most Catocala species. From my own observations it seems that late instar C. epione (Drury) and C. c. consors 0. E. Smith) larvae almost invariably rest on branches or small trunks. Late instars of Catocala habilis larvae are quite flattened and can be found resting under bark shags. -

STATES 00000000A F 24Hr Lawn Insect Killer 32Oz.RTS
92564-17_BioAdvanced SBS 24 hr Lawn Insect Killer_20170928_80_92564_.pdf 24 Hour Lawn Insect Killer READY-TO-SPRAY Mosquitoes KILLS Listed Insects in 24 Hours Ants Fleas KILLS Over * Ticks 500 Insects Chinch Bugs Mata los insectos de la lista en 24 horas Mata más de 500 insectos* Active Ingredients: Mole Crickets Cyfluthrin . 0.75% Other Ingredients . 99.25% Total 100.00% KEEP OUT OF REACH OF CHILDREN See back panel booklet for additional CAUTION precautionary statements *Listed insects NET CONTENTS 32 FL OZ (946ml) OPEN (continuación) Resealable Label VID 24 Hour for Directions & PARA MEJORES RESULTADOS: No regar las hojas dentro de una hora luego de la aplicación. Para evitar daños graves, eliminar las plagas Precautions comienzos de la temporada antes de que se multipliquen. Lawn Insect ® Las personas y las mascotas pueden volver a ingresar al área tratada una vez que se haya secado. Killer READY-TO-SPRAY This product kills listed outdoor pests by contact. Keeping your garden and lawn free of listed pests ALMACENAMIENTO Y ELIMINACIÓN has never been easier. This product is ready to spray. Just attach this bottle to your hose according to ALMACENAMIENTO DEL PESTICIDA directions. Water automatically mixes with the concentrate as you spray. Almacenar en el contenedor original y en un lugar fresco y seco, fuera del alcance de los niños, preferentemente en un armario bajo llave. READ THE LABEL FIRST! Indicaciones MANIPULACIÓN DEL CONTENEDOR en español Si está vacío: • Contenedor no reusable. No volver a rellenar ni utilizar este contenedor. • Colocar el contenedor vacío en la basura o entregarlo para QUICK FACTS después de las reciclar si fuera posible. -

Lepidoptera: Tortricidae: Tortricinae) and Evolutionary Correlates of Novel Secondary Sexual Structures
Zootaxa 3729 (1): 001–062 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Monograph ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3729.1.1 http://zoobank.org/urn:lsid:zoobank.org:pub:CA0C1355-FF3E-4C67-8F48-544B2166AF2A ZOOTAXA 3729 Phylogeny of the tribe Archipini (Lepidoptera: Tortricidae: Tortricinae) and evolutionary correlates of novel secondary sexual structures JASON J. DOMBROSKIE1,2,3 & FELIX A. H. SPERLING2 1Cornell University, Comstock Hall, Department of Entomology, Ithaca, NY, USA, 14853-2601. E-mail: [email protected] 2Department of Biological Sciences, University of Alberta, Edmonton, Canada, T6G 2E9 3Corresponding author Magnolia Press Auckland, New Zealand Accepted by J. Brown: 2 Sept. 2013; published: 25 Oct. 2013 Licensed under a Creative Commons Attribution License http://creativecommons.org/licenses/by/3.0 JASON J. DOMBROSKIE & FELIX A. H. SPERLING Phylogeny of the tribe Archipini (Lepidoptera: Tortricidae: Tortricinae) and evolutionary correlates of novel secondary sexual structures (Zootaxa 3729) 62 pp.; 30 cm. 25 Oct. 2013 ISBN 978-1-77557-288-6 (paperback) ISBN 978-1-77557-289-3 (Online edition) FIRST PUBLISHED IN 2013 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2013 Magnolia Press 2 · Zootaxa 3729 (1) © 2013 Magnolia Press DOMBROSKIE & SPERLING Table of contents Abstract . 3 Material and methods . 6 Results . 18 Discussion . 23 Conclusions . 33 Acknowledgements . 33 Literature cited . 34 APPENDIX 1. 38 APPENDIX 2. 44 Additional References for Appendices 1 & 2 . 49 APPENDIX 3. 51 APPENDIX 4. 52 APPENDIX 5. -

2010 Season Summary Index NEW WOFTHE~ Zone 1: Yukon Territory
2010 Season Summary Index NEW WOFTHE~ Zone 1: Yukon Territory ........................................................................................... 3 Alaska ... ........................................ ............................................................... 3 LEPIDOPTERISTS Zone 2: British Columbia .................................................... ........................ ............ 6 Idaho .. ... ....................................... ................................................................ 6 Oregon ........ ... .... ........................ .. .. ............................................................ 10 SOCIETY Volume 53 Supplement Sl Washington ................................................................................................ 14 Zone 3: Arizona ............................................................ .................................... ...... 19 The Lepidopterists' Society is a non-profo California ............... ................................................. .............. .. ................... 2 2 educational and scientific organization. The Nevada ..................................................................... ................................ 28 object of the Society, which was formed in Zone 4: Colorado ................................ ... ............... ... ...... ......................................... 2 9 May 1947 and formally constituted in De Montana .................................................................................................... 51 cember -

Butterflies and Moths of Centre County, Pennsylvania, United States
Heliothis ononis Flax Bollworm Moth Coptotriche aenea Blackberry Leafminer Argyresthia canadensis Apyrrothrix araxes Dull Firetip Phocides pigmalion Mangrove Skipper Phocides belus Belus Skipper Phocides palemon Guava Skipper Phocides urania Urania skipper Proteides mercurius Mercurial Skipper Epargyreus zestos Zestos Skipper Epargyreus clarus Silver-spotted Skipper Epargyreus spanna Hispaniolan Silverdrop Epargyreus exadeus Broken Silverdrop Polygonus leo Hammock Skipper Polygonus savigny Manuel's Skipper Chioides albofasciatus White-striped Longtail Chioides zilpa Zilpa Longtail Chioides ixion Hispaniolan Longtail Aguna asander Gold-spotted Aguna Aguna claxon Emerald Aguna Aguna metophis Tailed Aguna Typhedanus undulatus Mottled Longtail Typhedanus ampyx Gold-tufted Skipper Polythrix octomaculata Eight-spotted Longtail Polythrix mexicanus Mexican Longtail Polythrix asine Asine Longtail Polythrix caunus (Herrich-Schäffer, 1869) Zestusa dorus Short-tailed Skipper Codatractus carlos Carlos' Mottled-Skipper Codatractus alcaeus White-crescent Longtail Codatractus yucatanus Yucatan Mottled-Skipper Codatractus arizonensis Arizona Skipper Codatractus valeriana Valeriana Skipper Urbanus proteus Long-tailed Skipper Urbanus viterboana Bluish Longtail Urbanus belli Double-striped Longtail Urbanus pronus Pronus Longtail Urbanus esmeraldus Esmeralda Longtail Urbanus evona Turquoise Longtail Urbanus dorantes Dorantes Longtail Urbanus teleus Teleus Longtail Urbanus tanna Tanna Longtail Urbanus simplicius Plain Longtail Urbanus procne Brown Longtail -

CHECKLIST of WISCONSIN MOTHS (Superfamilies Mimallonoidea, Drepanoidea, Lasiocampoidea, Bombycoidea, Geometroidea, and Noctuoidea)
WISCONSIN ENTOMOLOGICAL SOCIETY SPECIAL PUBLICATION No. 6 JUNE 2018 CHECKLIST OF WISCONSIN MOTHS (Superfamilies Mimallonoidea, Drepanoidea, Lasiocampoidea, Bombycoidea, Geometroidea, and Noctuoidea) Leslie A. Ferge,1 George J. Balogh2 and Kyle E. Johnson3 ABSTRACT A total of 1284 species representing the thirteen families comprising the present checklist have been documented in Wisconsin, including 293 species of Geometridae, 252 species of Erebidae and 584 species of Noctuidae. Distributions are summarized using the six major natural divisions of Wisconsin; adult flight periods and statuses within the state are also reported. Examples of Wisconsin’s diverse native habitat types in each of the natural divisions have been systematically inventoried, and species associated with specialized habitats such as peatland, prairie, barrens and dunes are listed. INTRODUCTION This list is an updated version of the Wisconsin moth checklist by Ferge & Balogh (2000). A considerable amount of new information from has been accumulated in the 18 years since that initial publication. Over sixty species have been added, bringing the total to 1284 in the thirteen families comprising this checklist. These families are estimated to comprise approximately one-half of the state’s total moth fauna. Historical records of Wisconsin moths are relatively meager. Checklists including Wisconsin moths were compiled by Hoy (1883), Rauterberg (1900), Fernekes (1906) and Muttkowski (1907). Hoy's list was restricted to Racine County, the others to Milwaukee County. Records from these publications are of historical interest, but unfortunately few verifiable voucher specimens exist. Unverifiable identifications and minimal label data associated with older museum specimens limit the usefulness of this information. Covell (1970) compiled records of 222 Geometridae species, based on his examination of specimens representing at least 30 counties. -

An Annotated List of the Lepidoptera of Alberta, Canada
A peer-reviewed open-access journal ZooKeys 38: 1–549 (2010) Annotated list of the Lepidoptera of Alberta, Canada 1 doi: 10.3897/zookeys.38.383 MONOGRAPH www.pensoftonline.net/zookeys Launched to accelerate biodiversity research An annotated list of the Lepidoptera of Alberta, Canada Gregory R. Pohl1, Gary G. Anweiler2, B. Christian Schmidt3, Norbert G. Kondla4 1 Editor-in-chief, co-author of introduction, and author of micromoths portions. Natural Resources Canada, Northern Forestry Centre, 5320 - 122 St., Edmonton, Alberta, Canada T6H 3S5 2 Co-author of macromoths portions. University of Alberta, E.H. Strickland Entomological Museum, Department of Biological Sciences, Edmonton, Alberta, Canada T6G 2E3 3 Co-author of introduction and macromoths portions. Canadian Food Inspection Agency, Canadian National Collection of Insects, Arachnids and Nematodes, K.W. Neatby Bldg., 960 Carling Ave., Ottawa, Ontario, Canada K1A 0C6 4 Author of butterfl ies portions. 242-6220 – 17 Ave. SE, Calgary, Alberta, Canada T2A 0W6 Corresponding authors: Gregory R. Pohl ([email protected]), Gary G. Anweiler ([email protected]), B. Christian Schmidt ([email protected]), Norbert G. Kondla ([email protected]) Academic editor: Donald Lafontaine | Received 11 January 2010 | Accepted 7 February 2010 | Published 5 March 2010 Citation: Pohl GR, Anweiler GG, Schmidt BC, Kondla NG (2010) An annotated list of the Lepidoptera of Alberta, Canada. ZooKeys 38: 1–549. doi: 10.3897/zookeys.38.383 Abstract Th is checklist documents the 2367 Lepidoptera species reported to occur in the province of Alberta, Can- ada, based on examination of the major public insect collections in Alberta and the Canadian National Collection of Insects, Arachnids and Nematodes. -

Appendix 1- Species of Greatest Conservation Need Michigan’S Wildlife Action Plan 2015- 2025
Appendix 1- Species of Greatest Conservation Need Michigan’s Wildlife Action Plan 2015- 2025 Cover Photos Credits Habitat – MNFI, Yu Man Lee Cerulean Warbler – Roger Eriksson Category Common Name Scientific Name Inclusion Rationale Amphibians Blanchard’s cricket frog Acris crepitans Expert review (2005); blanchardi Technical Advisory Committee for the T&E list revision recommended this species to remain listed as Threatened (2014). There are current records across the species historical range, the southern third of the Lower Peninsula, but abundance is unknown and only historical records exist for several central counties. This species should stay listed as state threatened due to limited area of occupancy and declines. (TAC 2014) Amphibians Boreal chorus frog Pseudacris triseriata Expert review (2005); maculata Technical Advisory Committee for the T&E list revision recommended this species to remain listed as Special Concern (2014). The historical range of this species was restricted to Isle Royale; although the species appears to remain intact, abundance is unknown. Amphibians Marbled Salamander Ambystoma opacum Expert review (2005); Technical Advisory Committee for the T&E list revision recommended this species to be delisted from Endangered to Special Concern (2014). The historical range is limited to a small portion of the southwest Lower Peninsula and represents the northernmost extent of the species' range. The current distribution and abundance are not known, and no observations have been reported since the 1980s. It is unknown if this species is still present in Michigan as field research has been extremely limited in the small area of Category Common Name Scientific Name Inclusion Rationale known occupancy.