Feeding Biology of Cerambycids, Chapter 3
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LONGHORN BEETLE CHECKLIST - Beds, Cambs and Northants
LONGHORN BEETLE CHECKLIST - Beds, Cambs and Northants BCN status Conservation Designation/ current status Length mm In key? Species English name UK status Habitats/notes Acanthocinus aedilis Timberman Beetle o Nb 12-20 conifers, esp pine n ox-eye daisy and other coarse herbaceous plants [very recent Agapanthia cardui vr 6-14 n arrival in UK] Agapanthia villosoviridescens Golden-bloomed Grey LHB o f 10-22 mainly thistles & hogweed y Alosterna tabacicolor Tobacco-coloured LHB a f 6-8 misc deciduous, esp. oak, hazel y Anaglyptus mysticus Rufous-shouldered LHB o f Nb 6-14 misc trees and shrubs y Anastrangalia (Anoplodera) sanguinolenta r RDB3 9-12 Scots pine stumps n Anoplodera sexguttata Six-spotted LHB r vr RDB3 12-15 old oak and beech? n Anoplophora glabripennis Asian LHB vr introd 20-40 Potential invasive species n Arhopalus ferus (tristis) r r introd 13-25 pines n Arhopalus rusticus Dusky LHB o o introd 10-30 conifers y Aromia moschata Musk Beetle o f Nb 13-34 willows y Asemum striatum Pine-stump Borer o r introd 8-23 dead, fairly fresh pine stumps y Callidium violaceum Violet LHB r r introd 8-16 misc trees n Cerambyx cerdo ext ext introd 23-53 oak n Cerambyx scopolii ext introd 8-20 misc deciduous n Clytus arietus Wasp Beetle a a 6-15 misc, esp dead branches, posts y Dinoptera collaris r RDB1 7-9 rotten wood with other longhorns n Glaphyra (Molorchus) umbellatarum Pear Shortwing Beetle r o Na 5-8 misc trees & shrubs, esp rose stems y Gracilia minuta o r RDB2 2.5-7 woodland & scrub n Grammoptera abdominalis Black Grammoptera r r Na 6-9 -

Envenomations in Humans Caused by The
linica f C l To o x l ic a o n r l o u g o y J Amaral et al., J Clin Toxicol 2018, 8:4 Journal of Clinical Toxicology DOI: 10.4172/2161-0495.1000392 ISSN: 2161-0495 Case Report Open Access Envenomations in Humans Caused by the Venomous Beetle Onychocerus albitarsis: Observation of Two Cases in São Paulo State, Brazil Amaral ALS1*, Castilho AL1, Borges de Sá AL2 and Haddad V Jr3 1Departamento de Zoologia, Instituto de Biociências, Universidade Estadual Paulista – UNESP, CEP 18618-000, Botucatu, São Paulo State, Brazil 2Private Clinic, Botucatu, São Paulo State, Brazil 3Departamento de Dermatologia e Radioterapia, Faculdade de Medicina, Universidade Estadual Paulista – UNESP, CP 557, CEP 18618-000, Botucatu, São Paulo State, Brazil *Corresponding author: Antonio L. Sforcin Amaral, Departamento de Zoologia, Instituto de Biociências, Universidade Estadual Paulista – UNESP, CEP 18618-000, Botucatu, São Paulo State, Brazil, Email: [email protected] Received date: July 23, 2018; Accepted date: August 21, 2018; Published date: August 24, 2018 Copyright: ©2018 Amaral ALS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Beetles (Coleoptera) are the most diverse group of animals in the world and occur in many environments. In Atlantic and Amazon rainforests, the scorpion-beetle Onychocerus albitarsis (Cerambycidae), can be found. It has venom glandules and inoculators organs in the antenna extremities. Two injuries in humans are reported, showing different patterns of skin reaction after the stings. -

The Genus Canidia Thomson, 1857 (Coleoptera: Cerambycidae, Lamiinae, Acanthocinini)
Zootaxa 927: 1–27 (2005) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA 927 Copyright © 2005 Magnolia Press ISSN 1175-5334 (online edition) The genus Canidia Thomson, 1857 (Coleoptera: Cerambycidae, Lamiinae, Acanthocinini) JAMES E. WAPPES¹ & STEVEN W. LINGAFELTER² ¹ American Coleoptera Museum, 179 Fall Creek, Bulverde, TX 78163 U. S. A. [email protected] ² Systematic Entomology Lab, Plant Sciences Institute, Agriculture Research Service, USDA, National Museum of Natural History, Smithsonian Institution, MRC-168, Washington, DC 20013-7012 U. S. A. [email protected] Abstract The lamiine genus Canidia Thomson is redefined with Canidiopsis Dillon and Pseudocanidia Dil- lon as new synonyms. Three new species from Mexico are described and illustrated: Canidia chemsaki, C. giesberti, and C. turnbowi. The following new synonymies are proposed: Canidiop- sis similis Dillon, 1955 and Canidiopsis hebes Dillon, 1955 = Canidia mexicana Thomson, 1860; Pseudocanidia cuernavacae Dillon, 1955 = Dectes spinicornis Bates, 1881; and Dectes (Canidia) balteata var. inapicalis Tippmann, 1960 = Dectes balteatus Lacordaire, 1872. A key to the eight species and one subspecies is presented. Key words: Insecta, Coleoptera, Cerambycidae, Lamiinae, Acanthocinini, Canidia, Dectes, Can- idiopsis, Pseudocanidia, new species, key Resumen: Se redefine el género Canidia Thomson con Canidiopsis Dillon y Pseudocanidia Dillon como sinónimos nuevos. Describimos e ilustramos tres especies nuevas de México: Canidia chemsaki, C. giesberti y C. turnbowi. Se proponen los siguientes sinónimos nuevos: Canidiopsis similis Dillon, 1955 y Canidiopsis hebes Dillon 1955 = Canidia mexicana Thomson, 1860; Pseudocanidia cuernavacae Dillon, 1955 = Dectes spinicornis Bates, 1881; y Dectes (Canidia) balteata inapicalis Tippmann, 1960 = Dectes balteatus Lacordaire, 1872. Se incluye una clave para separar las ocho especies y una subespecie. -

(Coleoptera) of Peru Miguel A
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Center for Systematic Entomology, Gainesville, Insecta Mundi Florida 2-29-2012 Preliminary checklist of the Cerambycidae, Disteniidae, and Vesperidae (Coleoptera) of Peru Miguel A. Monné Universidade Federal do Rio de Janeiro, [email protected] Eugenio H. Nearns University of New Mexico, [email protected] Sarah C. Carbonel Carril Universidad Nacional Mayor de San Marcos, Peru, [email protected] Ian P. Swift California State Collection of Arthropods, [email protected] Marcela L. Monné Universidade Federal do Rio de Janeiro, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/insectamundi Part of the Entomology Commons Monné, Miguel A.; Nearns, Eugenio H.; Carbonel Carril, Sarah C.; Swift, Ian P.; and Monné, Marcela L., "Preliminary checklist of the Cerambycidae, Disteniidae, and Vesperidae (Coleoptera) of Peru" (2012). Insecta Mundi. Paper 717. http://digitalcommons.unl.edu/insectamundi/717 This Article is brought to you for free and open access by the Center for Systematic Entomology, Gainesville, Florida at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Insecta Mundi by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. INSECTA MUNDI A Journal of World Insect Systematics 0213 Preliminary checklist of the Cerambycidae, Disteniidae, and Vesperidae (Coleoptera) of Peru Miguel A. Monné Museu Nacional Universidade Federal do Rio de Janeiro Quinta da Boa Vista São Cristóvão, 20940-040 Rio de Janeiro, RJ, Brazil Eugenio H. Nearns Department of Biology Museum of Southwestern Biology University of New Mexico Albuquerque, NM 87131-0001, USA Sarah C. Carbonel Carril Departamento de Entomología Museo de Historia Natural Universidad Nacional Mayor de San Marcos Avenida Arenales 1256, Lima, Peru Ian P. -

New Longhorn Beetles (Coleoptera: Cerambycidae) from Serbia
Arch. Biol. Sci., Belgrade, 57 (4), 27P-28P, 2005. NEW LONGHORN BEETLES (COLEOPTERA: CERAMBYCIDAE) FROM SERBIA. Nataša Pil1 and D. Stojanović2. 1Institute for Nature Conservation of Serbia, 21000 Novi Sad, Serbia and Montenegro, 2”Fruška Gora” National Park, 21208 Sremska Kamenica, Serbia and Montenegro UDC 597.76(497.11) Since the 1980’s, longhorn beetles (Coleoptera, Cerambycidae) They feed in the central region of the cone or occasionally in have been only randomly researched in Serbia. From earlier the base of old scales. The life cycle probably last two years, years, there are very detailed publications on this insect group and pupation very likely occurs in the soil. Adults emerge in (A d a m o v i ć , 1965; M i k š i ć and G e o r g i j e v i ć , 1971; April-July, on flowers. The given species differs from the simi- 1973; M i k š i ć and K o r p i č , 1985). lar Cortodera humeralis (Schaller, 1783) in having only sparse pubescence on the pronotum and head, with glabrous median The most recent data (I l i ć , 2005) indicate the presence line, and sparse pubescence on the outer border of the eye and of 245 longhorn beetle species (Coleoptera: Cerambycidae) in base of the antennae. Serbia. Not included in the mentioned publication, the follow- ing five species should be added to the list: Cortodera discolor 3. Vadonia hirsuta (Daniel and Daniel,1891) Fairmaire, 1866; Stenopterus similatus Holzschuh, 1979; Chlo- rophorus aegyptiacus (Fabricius, 1775); Agapanthia osmanlis (New data: Mt. -

Coleoptera, Cerambycidae, Lamiinae) with Description of a Species with Non‑Retractile Parameres
ARTICLE Two new genera of Desmiphorini (Coleoptera, Cerambycidae, Lamiinae) with description of a species with non‑retractile parameres Francisco Eriberto de Lima Nascimento¹² & Antonio Santos-Silva¹³ ¹ Universidade de São Paulo (USP), Museu de Zoologia (MZUSP). São Paulo, SP, Brasil. ² ORCID: http://orcid.org/0000-0002-5047-8921. E-mail: [email protected] ³ ORCID: http://orcid.org/0000-0001-7128-1418. E-mail: [email protected] Abstract. In this study, two new genera of Desmiphorini (Lamiinae) are proposed: Cleidaria gen. nov., to include Cleidaria cleidae sp. nov. from the state of Chiapas in Mexico, and Obscenoides gen. nov. for Desmiphora (D.) compta Martins & Galileo, 2005. The shape of tarsal claws of Cleidaria cleidae sp. nov. (abruptly narrowed from basal half) is so far, not found in any current genera of the tribe. With respect to Obscenoides compta (Martins & Galileo, 2005) comb. nov., the genitalia of males have an unusual shape with non-retractile parameres. The character combination related to this genital structure is unknown to us in other species in the family, and hypotheses about its function are suggested. Key-Words. Genital morphology; Longhorned beetles; New taxa; Taxonomy. INTRODUCTION the current definitions of some tribes, especially based on the works of Breuning do not take into Lamiinae (Cerambycidae), also known as flat- account adaptive convergences and use superfi- faced longhorns, with more than 21,000 described cial characters to subordinate taxa. species in about 3,000 genera and 87 tribes is Among these tribes, Desmiphorini Thomson, the largest subfamily of Cerambycidae occurring 1860 is not an exception, and its “boundaries” are worldwide (Tavakilian & Chevillotte, 2019). -

Ethanol and (–)-A-Pinene: Attractant Kairomones for Some Large Wood-Boring Beetles in Southeastern USA
J Chem Ecol (2006) DOI 10.1007/s10886-006-9037-8 Ethanol and (–)-a-Pinene: Attractant Kairomones for Some Large Wood-Boring Beetles in Southeastern USA Daniel R. Miller Received: 12 September 2005 /Revised: 12 December 2005 /Accepted: 2 January 2006 # Springer Science + Business Media, Inc. 2006 Abstract Ethanol and a-pinene were tested as attractants for large wood-boring pine beetles in Alabama, Florida, Georgia, North Carolina, and South Carolina in 2002–2004. Multiple-funnel traps baited with (j)-a-pinene (released at about 2 g/d at 25–28-C) were attractive to the following Cerambycidae: Acanthocinus nodosus, A. obsoletus, Arhopalus rusticus nubilus, Asemum striatum, Monochamus titillator, Prionus pocularis, Xylotrechus integer, and X. sagittatus sagittatus. Buprestis lineata (Buprestidae), Alaus myops (Elateridae), and Hylobius pales and Pachylobius picivorus (Curculionidae) were also attracted to traps baited with (j)-a-pinene. In many locations, ethanol synergized attraction of the cerambycids Acanthocinus nodosus, A. obsoletus, Arhopalus r. nubilus, Monochamus titillator, and Xylotrechus s. sagittatus (but not Asemum striatum, Prionus pocularis,orXylotrechus integer)to traps baited with (j)-a-pinene. Similarly, attraction of Alaus myops, Hylobius pales, and Pachylobius picivorus (but not Buprestis lineata) to traps baited with (j)-a- pinene was synergized by ethanol. These results provide support for the use of traps baited with ethanol and (j)-a-pinene to detect and monitor common large wood- boring beetles from the southeastern region of the USA at ports-of-entry in other countries, as well as forested areas in the USA. Keywords Cerambycidae . Xylotrechus . Monochamus . Acanthocinus Curculionidae . Hylobius . Pachylobius . Elateridae . Alaus . Ethanol a-Pinene . -

25Th U.S. Department of Agriculture Interagency Research Forum On
US Department of Agriculture Forest FHTET- 2014-01 Service December 2014 On the cover Vincent D’Amico for providing the cover artwork, “…and uphill both ways” CAUTION: PESTICIDES Pesticide Precautionary Statement This publication reports research involving pesticides. It does not contain recommendations for their use, nor does it imply that the uses discussed here have been registered. All uses of pesticides must be registered by appropriate State and/or Federal agencies before they can be recommended. CAUTION: Pesticides can be injurious to humans, domestic animals, desirable plants, and fish or other wildlife--if they are not handled or applied properly. Use all pesticides selectively and carefully. Follow recommended practices for the disposal of surplus pesticides and pesticide containers. Product Disclaimer Reference herein to any specific commercial products, processes, or service by trade name, trademark, manufacturer, or otherwise does not constitute or imply its endorsement, recom- mendation, or favoring by the United States government. The views and opinions of wuthors expressed herein do not necessarily reflect those of the United States government, and shall not be used for advertising or product endorsement purposes. The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, sex, religion, age, disability, political beliefs, sexual orientation, or marital or family status. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at 202-720-2600 (voice and TDD). To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, Room 326-W, Whitten Building, 1400 Independence Avenue, SW, Washington, D.C. -
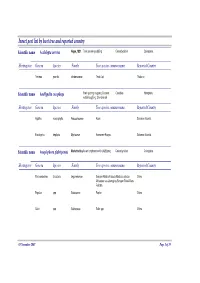
Insect Pest List by Host Tree and Reported Country
Insect pest list by host tree and reported country Scientific name Acalolepta cervina Hope, 1831 Teak canker grub|Eng Cerambycidae Coleoptera Hosting tree Genera Species Family Tree species common name Reported Country Tectona grandis Verbenaceae Teak-Jati Thailand Scientific name Amblypelta cocophaga Fruit spotting bug|eng Coconut Coreidae Hemiptera nutfall bug|Eng, Chinche del Hosting tree Genera Species Family Tree species common name Reported Country Agathis macrophylla Araucariaceae Kauri Solomon Islands Eucalyptus deglupta Myrtaceae Kamarere-Bagras Solomon Islands Scientific name Anoplophora glabripennis Motschulsky Asian longhorn beetle (ALB)|eng Cerambycidae Coleoptera Hosting tree Genera Species Family Tree species common name Reported Country Paraserianthes falcataria Leguminosae Sengon-Albizia-Falcata-Molucca albizia- China Moluccac sau-Jeungjing-Sengon-Batai-Mara- Falcata Populus spp. Salicaceae Poplar China Salix spp. Salicaceae Salix spp. China 05 November 2007 Page 1 of 35 Scientific name Aonidiella orientalis Newstead, Oriental scale|eng Diaspididae Homoptera 1894 Hosting tree Genera Species Family Tree species common name Reported Country Lovoa swynnertonii Meliaceae East African walnut Cameroon Azadirachta indica Meliaceae Melia indica-Neem Nigeria Scientific name Apethymus abdominalis Lepeletier, Tenthredinidae Hymenoptera 1823 Hosting tree Genera Species Family Tree species common name Reported Country Other Coniferous Other Coniferous Romania Scientific name Apriona germari Hope 1831 Long-horned beetle|eng Cerambycidae -

Histoires Naturelles N°16 Histoires Naturelles N°16
Histoires Naturelles n°16 Histoires Naturelles n°16 Essai de liste des Coléoptères de France Cyrille Deliry - Avril 2011 ! - 1 - Histoires Naturelles n°16 Essai de liste des Coléoptères de France Les Coléoptères forment l"ordre de plus diversifié de la Faune avec près de 400000 espèces indiquées dans le Monde. On en compte près de 20000 en Europe et pus de 9600 en France. Classification des Coléoptères Lawrence J.F. & Newton A.F. 1995 - Families and subfamilies of Coleoptera (with selected genera, notes, references and data on family-group names) In : Biology, Phylogeny, and Classification of Coleoptera. - éd. J.Pakaluk & S.A Slipinski, Varsovie : 779-1006. Ordre Coleoptera Sous-ordre Archostemata - Fam. Ommatidae, Crowsoniellidae, Micromathidae, Cupedidae Sous-ordre Myxophaga - Fam. Lepiceridae, Torridincolidae, Hydroscaphidae, Microsporidae Sous-ordre Adephaga - Fam. Gyrinidae, Halipidae, Trachypachidae, Noteridae, Amphizoidae, Hygrobiidae, Dytiscidae, Rhysodidae, Carabidae (Carabinae, Cicindelinae, Trechinae...) Sous-ordre Polyphaga Série Staphyliniformia - Superfam. Hydrophyloidea, Staphylinoidea Série Scarabaeiformia - Fam. Lucanidae, Passalidae, Trogidae, Glaresidae, Pleocmidae, Diphyllostomatidae, Geotrupidae, Belohinidae, Ochodaeidae, Ceratocanthidae, Hybrosoridae, Glaphyridae, Scarabaridea (Scarabaeinae, Melolonthinae, Cetoniinae...) Série Elateriformia - Superfam. Scirtoidea, Dascilloidea, Buprestoidea (Buprestidae), Byrrhoidea, Elateroidea (Elateridae, Lampyridae, Cantharidae...) + Incertae sedis - Fam. Podabrocephalidae, Rhinophipidae -

Scope: Munis Entomology & Zoology Publishes a Wide Variety of Papers
_____________ Mun. Ent. Zool. Vol. 4, No. 1, January 2009___________ I MUNIS ENTOMOLOGY & ZOOLOGY Ankara / Turkey II _____________ Mun. Ent. Zool. Vol. 4, No. 1, January 2009___________ Scope: Munis Entomology & Zoology publishes a wide variety of papers on all aspects of Entomology and Zoology from all of the world, including mainly studies on systematics, taxonomy, nomenclature, fauna, biogeography, biodiversity, ecology, morphology, behavior, conservation, paleobiology and other aspects are appropriate topics for papers submitted to Munis Entomology & Zoology. Submission of Manuscripts: Works published or under consideration elsewhere (including on the internet) will not be accepted. At first submission, one double spaced hard copy (text and tables) with figures (may not be original) must be sent to the Editors, Dr. Hüseyin Özdikmen for publication in MEZ. All manuscripts should be submitted as Word file or PDF file in an e-mail attachment. If electronic submission is not possible due to limitations of electronic space at the sending or receiving ends, unavailability of e-mail, etc., we will accept “hard” versions, in triplicate, accompanied by an electronic version stored in a floppy disk, a CD-ROM. Review Process: When submitting manuscripts, all authors provides the name, of at least three qualified experts (they also provide their address, subject fields and e-mails). Then, the editors send to experts to review the papers. The review process should normally be completed within 45-60 days. After reviewing papers by reviwers: Rejected papers are discarded. For accepted papers, authors are asked to modify their papers according to suggestions of the reviewers and editors. Final versions of manuscripts and figures are needed in a digital format. -

Biosecurity Regulation 2016
Queensland Biosecurity Act 2014 Biosecurity Regulation 2016 Current as at 14 August 2020 © State of Queensland 2020 This work is licensed under a Creative Commons Attribution 4.0 International License. Queensland Biosecurity Regulation 2016 Contents Page Chapter 1 Preliminary 1 Short title . 11 2 Commencement . 11 3 Definitions . 11 3A Measurement of position under regulation . 11 Chapter 2 Biosecurity obligations Part 2 Codes of practice Division 1 Labelling of fertilisers and contaminants in fertilisers 6 Code of practice about labelling of fertilisers and contaminants in fertilisers—Act, s 104(1) . 12 7 Effect of code of practice—Act, s 26(1) . 12 Division 2 Feed for food producing animals 8 Code of practice for feed about food producing animals—Act, s 104(1) 13 9 Effect of code of practice—Act, s 26(1) . 13 Part 3 Obligations relating to restricted matter Division 1 Category 3 restricted matter Subdivision 1 Ways for disposing category 3 restricted matter 10 Object of subdivision . 14 11 Ways of disposing of category 3 restricted matter—invasive plants 14 11A Ways of disposing of category 3 restricted matter—invasive animals 15 Subdivision 1A Purposes for disposing of category 3 restricted matter 11B Object of subdivision . 15 11C Disposing of category 3 restricted matter—purpose authorised under another law . 15 Subdivision 1B Ways for distributing category 3 restricted matter 11D Object of subdivision . 15 11E Distributing category 3 restricted matter—way authorised under another Biosecurity Regulation 2016 Contents law . 15 Subdivision 2 Purposes for distributing category 3 restricted matter 12 Object of subdivision . 16 13 Distributing category 3 restricted matter—biological control .