The Manhattan Project
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Taming of “49” Big Science in Little Time
The Taming of “49” Big science in little time Recollections of Edward F. Hammel During the Manhattan Project, plutonium was often referred to, simply, as 49. Number 4 was for the last digit in 94 (the atomic number of plutonium) and 9 for the last digit in plutonium-239, the isotope of choice for nuclear weapons. The story that unfolds was adapted from Plutonium Metallurgy at Los Alamos, 1943–1945, as Edward F. Hammel remembers the events of those years. 48 Los Alamos Science Number 26 2000 The Taming of “49” he work in plutonium chemistry tion work was an inevitable conse- the metal could be fabricated into and metallurgy carried out at quence of the nuclear and physical satisfactory weapon components. TLos Alamos (Site Y) between research that was still to be conducted In addition, not until January 1944 1943 and 1945 had a somewhat contro- on the metal. It would clearly have did the first few milligrams of pile- versial history. The controversy was been inefficient and time consuming to produced plutonium arrive at Los about who was going to do what. ship small amounts of plutonium metal Alamos. The first 1-gram shipment At the time Los Alamos was being back to Chicago for repurification and arrived in February 1944, and quantity organized, most of the expertise in plu- refabrication into different sizes and shipments of plutonium did not begin to tonium chemistry resided at Berkeley, shapes for the next-scheduled nuclear arrive at Los Alamos until May 1945. where plutonium was discovered in physics experiment. From the outset, it was clear that the December 1940, and at the Met Lab in Minimizing the time spent to solve purification of plutonium was the most Chicago. -

2012 04 Newsletter
United States Atmospheric & Underwater Atomic Weapon Activities National Association of Atomic Veterans, Inc. 1945 “TRINITY“ “Assisting America’s Atomic Veterans Since 1979” ALAMOGORDO, N. M. Website: www.naav.com E-mail: [email protected] 1945 “LITTLE BOY“ HIROSHIMA, JAPAN R. J. RITTER - Editor April, 2012 1945 “FAT MAN“ NAGASAKI, JAPAN 1946 “CROSSROADS“ BIKINI ISLAND 1948 “SANDSTONE“ ENEWETAK ATOLL 1951 “RANGER“ NEVADA TEST SITE 1951 “GREENHOUSE“ ENEWETAK ATOLL 1951 “BUSTER – JANGLE“ NEVADA TEST SITE 1952 “TUMBLER - SNAPPER“ NEVADA TEST SITE 1952 “IVY“ ENEWETAK ATOLL 1953 “UPSHOT - KNOTHOLE“ NEVADA TEST SITE 1954 “CASTLE“ BIKINI ISLAND 1955 “TEAPOT“ NEVADA TEST SITE 1955 “WIGWAM“ OFFSHORE SAN DIEGO 1955 “PROJECT 56“ NEVADA TEST SITE 1956 “REDWING“ ENEWETAK & BIKINI 1957 “PLUMBOB“ NEVADA TEST SITE 1958 “HARDTACK-I“ ENEWETAK & BIKINI 1958 “NEWSREEL“ JOHNSTON ISLAND 1958 “ARGUS“ SOUTH ATLANTIC 1958 “HARDTACK-II“ NEVADA TEST SITE 1961 “NOUGAT“ NEVADA TEST SITE 1962 “DOMINIC-I“ CHRISTMAS ISLAND JOHNSTON ISLAND 1965 “FLINTLOCK“ AMCHITKA, ALASKA 1969 “MANDREL“ AMCHITKA, ALASKA 1971 “GROMMET“ AMCHITKA, ALASKA 1974 “POST TEST EVENTS“ ENEWETAK CLEANUP ------------ “ IF YOU WERE THERE, YOU ARE AN ATOMIC VETERAN “ The Newsletter for America’s Atomic Veterans COMMANDER’S COMMENTS knowing the seriousness of the situation, did not register any Outreach Update: First, let me extend our discomfort, or dissatisfaction on her part. As a matter of fact, it thanks to the membership and friends of NAAV was kind of nice to have some of those callers express their for supporting our “outreach” efforts over the thanks for her kind attention and assistance. We will continue past several years. It is that firm dedication to to insure that all inquires, along these lines, are fully and our Mission-Statement that has driven our adequately addressed. -
Laser Isotope Separation (LIS), Technical and Economic
NASA TECHNICAL MEMORANDUM A STATUS OF PROGRESS FOR THL LASER lsofopE SEPARATION (11 SI PROCESS +tear 1976 NASA George C. Mdr~bdlSpace Flight Center Marshdl Space Fb$t Center, Alabama lLSFC - Form 3190 (Rev June 1971) REPORT STANDARD TITLE PACE I nEPMTn0. 3. RECIPIENT*$ CATILOC NO. NASA TM X-73345 10 TITLE UO SUTlTLt IS. REPORT DATE I September A st.tUaof for Iaser isotOpe ¶tian lS76 I Progress the (LIS) 6 PERFWYIIIG WGUIZATIO* CQOE George C. M8ralmll!3gam Flight Center I 1. COUTRUT OR am yo. I MarW Flight Center, Alabama 35812 Tecbnid Memormdum National Aemutics and Space Administration Washingtan, D.C. 20546 I I Prepared by Systems Aaalysis and Integration Iaboratory, Science and Engineering An overview of the various categories of the LE3 methodology is given together with illustrations showing a simplified version of the LIS tecbnique, an example of the two-phoiin photoionization category, and a diagram depicting how the energy levels of various isdope influence the LIS process. A&icatlons have been proposed for the LIS system which, in addition to the use to enrich uranium, could in themselves develop into programs of tremendous scope and breadth. Such applications as treatment of radioac '--ewastes from light-water nKzlear reactors, enriching the deuterlum isotope to make heavv-water, and enrlchhg tik light isotopes of such 17 KEt WORDS 18. DISTRIBUTION STATEMENT 5ECUQlTY CLASSIF. Ff thh PI*) 21 NO. OF PAbFS 22 PRICE Unclassified Unclassified I 20 NTIS PREFACE Since the publication of t& first Techid hiemomxitun (TM X-64947) on the Laser hotope Separation (LE)process in May 1975 [l], there bbeen a virtual explosion of available information on this process. -

Hitler and Heisenberg
The Wall Street Journal December 27-28, 2014 Book Review “Serving the Reich” by Philip Ball, Chicago, $30, 303 pages Hitler and Heisenberg Werner Heisenberg thought that once Germany had won, the ‘good Germans’ would get rid of the Nazis. Fizzing The museum inside the former basement tavern where in early 1945 German scientists built a small nuclear reactor. Associated Press by Jeremy Bernstein The German scientists who remained in Germany throughout the Nazi period can be divided into three main groups. At one end were the anti-Nazis—people who not only despised the regime but tried to do something about it. They were very few. Two that come to mind were Fritz Strassmann and Max von Laue. Strassmann was a radiochemist—a specialist in radioactive materials—who was blacklisted from university jobs by the Society of German Chemists in 1933 for his anti-Nazi views. He was fortunate to get a half-pay job at the Kaiser Wilhelm Institute for Chemistry, which got some of its funding from industry. He and his wife (they had a 3-year-old son) hid a Jewish friend in their apartment for months. With the radiochemist Otto Hahn, he performed the 1938 experiment in which fission was observed for the first time. Von Laue won the Nobel Prize in physics in 1914. Soon after Einstein published his 1905 paper on relativity, von Laue went to Switzerland to see him. They remained friends for life. Under the Nazis, Einstein’s work was denounced as “Jewish science.” When a number of von Laue’s colleagues agreed to use relativity but attribute it to some Aryan, he refused. -

{PDF EPUB} the Manhattan Projects Vol. 5 the Cold War by Jonathan Hickman the Irreverence of the MANHATTAN PROJECTS
Read Ebook {PDF EPUB} The Manhattan Projects Vol. 5 The Cold War by Jonathan Hickman The Irreverence of THE MANHATTAN PROJECTS. Jonathan Hickman’s and Nick Pitarra’s THE MANHATTAN PROJECTS (2012-2015) depicts national heroes American, German, and Russian alike as violent and self-absorbed madmen. It elevates to superhuman levels the genius attributed to some of them, and deepens to subhuman levels the depravity we naturally suspect prodigious men of flirting with. Whenever we ask the question, “How can Franklin Delano Roosevelt, or Joseph Robert Oppenheimer, or SS Major Wernher Magnus Maximilian, Herr Baron von Braun, PhD., have accomplished so much in a single lifetime?” one of the answers we unconsciously consider is that they were unhinged, power-mad, driven by forces most of us never feel. If they slept less, thought more, worked longer hours, and pushed their ideas through opposition with more commitment than the rest of us, then did their private passions and their consciences also behave differently from those of your everyday mere mortal? THE MANHATTAN PROJECTS’ cast includes four Presidents of the United States—FDR, Harry S. Truman, JFK, and LBJ—two US Army generals— Leslie Groves and William Westmoreland —four famous scientists from one-time facist European states—Albert Einstein, Enrico Fermi, Wernher von Braun, and Helmutt Gröttrup — three famous American physicists—J. Robert Oppenheimer, Richard Feynman, and Harry Daghlian —one Defense Minister of the USSR and one of its eventual Premiers— Dmitriy Ustinov and Leonid Brezhnev—and two of the earliest cosmonauts—Yuri Gagarin and Laika , a dog. But it is not history. -

The Making of an Atomic Bomb
(Image: Courtesy of United States Government, public domain.) INTRODUCTORY ESSAY "DESTROYER OF WORLDS": THE MAKING OF AN ATOMIC BOMB At 5:29 a.m. (MST), the world’s first atomic bomb detonated in the New Mexican desert, releasing a level of destructive power unknown in the existence of humanity. Emitting as much energy as 21,000 tons of TNT and creating a fireball that measured roughly 2,000 feet in diameter, the first successful test of an atomic bomb, known as the Trinity Test, forever changed the history of the world. The road to Trinity may have begun before the start of World War II, but the war brought the creation of atomic weaponry to fruition. The harnessing of atomic energy may have come as a result of World War II, but it also helped bring the conflict to an end. How did humanity come to construct and wield such a devastating weapon? 1 | THE MANHATTAN PROJECT Models of Fat Man and Little Boy on display at the Bradbury Science Museum. (Image: Courtesy of Los Alamos National Laboratory.) WE WAITED UNTIL THE BLAST HAD PASSED, WALKED OUT OF THE SHELTER AND THEN IT WAS ENTIRELY SOLEMN. WE KNEW THE WORLD WOULD NOT BE THE SAME. A FEW PEOPLE LAUGHED, A FEW PEOPLE CRIED. MOST PEOPLE WERE SILENT. J. ROBERT OPPENHEIMER EARLY NUCLEAR RESEARCH GERMAN DISCOVERY OF FISSION Achieving the monumental goal of splitting the nucleus The 1930s saw further development in the field. Hungarian- of an atom, known as nuclear fission, came through the German physicist Leo Szilard conceived the possibility of self- development of scientific discoveries that stretched over several sustaining nuclear fission reactions, or a nuclear chain reaction, centuries. -

The Manhattan Project and Its Legacy
Transforming the Relationship between Science and Society: The Manhattan Project and Its Legacy Report on the workshop funded by the National Science Foundation held on February 14 and 15, 2013 in Washington, DC Table of Contents Executive Summary iii Introduction 1 The Workshop 2 Two Motifs 4 Core Session Discussions 6 Scientific Responsibility 6 The Culture of Secrecy and the National Security State 9 The Decision to Drop the Bomb 13 Aftermath 15 Next Steps 18 Conclusion 21 Appendix: Participant List and Biographies 22 Copyright © 2013 by the Atomic Heritage Foundation. All rights reserved. No part of this book, either text or illustration, may be reproduced or transmit- ted in any form by any means, electronic or mechanical, including photocopying, reporting, or by any information storage or retrieval system without written persmission from the publisher. Report prepared by Carla Borden. Design and layout by Alexandra Levy. Executive Summary The story of the Manhattan Project—the effort to develop and build the first atomic bomb—is epic, and it continues to unfold. The decision by the United States to use the bomb against Japan in August 1945 to end World War II is still being mythologized, argued, dissected, and researched. The moral responsibility of scientists, then and now, also has remained a live issue. Secrecy and security practices deemed necessary for the Manhattan Project have spread through the govern- ment, sometimes conflicting with notions of democracy. From the Manhattan Project, the scientific enterprise has grown enormously, to include research into the human genome, for example, and what became the Internet. Nuclear power plants provide needed electricity yet are controversial for many people. -

Richard G. Hewlett and Jack M. Holl. Atoms
ATOMS PEACE WAR Eisenhower and the Atomic Energy Commission Richard G. Hewlett and lack M. Roll With a Foreword by Richard S. Kirkendall and an Essay on Sources by Roger M. Anders University of California Press Berkeley Los Angeles London Published 1989 by the University of California Press Berkeley and Los Angeles, California University of California Press, Ltd. London, England Prepared by the Atomic Energy Commission; work made for hire. Library of Congress Cataloging-in-Publication Data Hewlett, Richard G. Atoms for peace and war, 1953-1961. (California studies in the history of science) Bibliography: p. Includes index. 1. Nuclear energy—United States—History. 2. U.S. Atomic Energy Commission—History. 3. Eisenhower, Dwight D. (Dwight David), 1890-1969. 4. United States—Politics and government-1953-1961. I. Holl, Jack M. II. Title. III. Series. QC792. 7. H48 1989 333.79'24'0973 88-29578 ISBN 0-520-06018-0 (alk. paper) Printed in the United States of America 1 2 3 4 5 6 7 8 9 CONTENTS List of Illustrations vii List of Figures and Tables ix Foreword by Richard S. Kirkendall xi Preface xix Acknowledgements xxvii 1. A Secret Mission 1 2. The Eisenhower Imprint 17 3. The President and the Bomb 34 4. The Oppenheimer Case 73 5. The Political Arena 113 6. Nuclear Weapons: A New Reality 144 7. Nuclear Power for the Marketplace 183 8. Atoms for Peace: Building American Policy 209 9. Pursuit of the Peaceful Atom 238 10. The Seeds of Anxiety 271 11. Safeguards, EURATOM, and the International Agency 305 12. -
Grappling with the Bomb: Britain's Pacific H-Bomb Tests
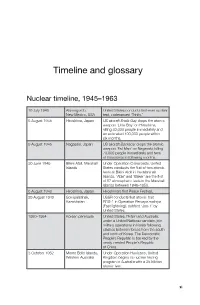
Timeline and glossary Nuclear timeline, 1945–1963 16 July 1945 Alamogordo, United States conducts first-ever nuclear New Mexico, USA test, codenamed ‘Trinity .’ 6 August 1945 Hiroshima, Japan US aircraft Enola Gay drops the atomic weapon ‘Little Boy’ on Hiroshima, killing 80,000 people immediately and an estimated 100,000 people within six months . 9 August 1945 Nagasaki, Japan US aircraft Bockscar drops the atomic weapon ‘Fat Man’ on Nagasaki, killing 70,000 people immediately and tens of thousands in following months . 30 June 1946 Bikini Atoll, Marshall Under Operation Crossroads, United Islands States conducts the first of two atomic tests at Bikini Atoll in the Marshall Islands. ‘Able’ and ‘Baker’ are the first of 67 atmospheric tests in the Marshall Islands between 1946–1958 . 6 August 1948 Hiroshima, Japan Hiroshima’s first Peace Festival. 29 August 1949 Semipalatinsk, USSR conducts first atomic test Kazakhstan RDS-1 in Operation Pervaya molniya (Fast lightning), dubbed ‘Joe-1’ by United States . 1950–1954 Korean peninsula United States, Britain and Australia, under a United Nations mandate, join military operations in Korea following clashes between forces from the south and north of Korea. The Democratic People’s Republic is backed by the newly created People’s Republic of China . 3 October 1952 Monte Bello Islands, Under Operation Hurricane, United Western Australia Kingdom begins its nuclear testing program in Australia with a 25 kiloton atomic test . xi GRAPPLING WITH THE BOMB 1 November 1952 Bikini Atoll, Marshall United States conducts its first Islands hydrogen bomb test, codenamed ‘Mike’ (10 .4 megatons) as part of Operation Ivy . -

Trinity Scientific Firsts
TRINITY SCIENTIFIC FIRSTS THE TRINITY TEST was perhaps the greatest scientifi c experiment ever. Seventy-fi ve years ago, Los Alamos scientists and engineers from the U.S., Britain, and Canada changed the world. July 16, 1945 marks the entry into the Atomic Age. PLUTONIUM: THE BETHE-FEYNMAN FORMULA: Scientists confi rmed the newly discovered 239Pu has attractive nuclear Nobel Laureates Hans Bethe and Richard Feynman developed the physics fi ssion properties for an atomic weapon. They were able to discern which equation used to estimate the yield of a fi ssion weapon, building on earlier production path would be most effective based on nuclear chemistry, and work by Otto Frisch and Rudolf Peierls. The equation elegantly encapsulates separated plutonium from Hanford reactor fuel. essential physics involved in the nuclear explosion process. PRECISION HIGH-EXPLOSIVE IMPLOSION FOUNDATIONAL RADIOCHEMICAL TO CREATE A SUPER-CRITICAL ASSEMBLY: YIELD ANALYSIS: Project Y scientists developed simultaneously-exploding bridgewire detonators Wartime radiochemistry techniques developed and used at Trinity with a pioneering high-explosive lens system to create a symmetrically provide the foundation for subsequent analyses of nuclear detonations, convergent detonation wave to compress the core. both foreign and domestic. ADVANCED IMAGING TECHNIQUES: NEW FRONTIERS IN COMPUTING: Complementary diagnostics were developed to optimize the implosion Human computers and IBM punched-card machines together calculated design, including fl ash x-radiography, the RaLa method, -

The Effect of Chemical Incidents on First Responders
2019 The Effect of Chemical Incidents on First Responders: An Interview with Bruce Evans (MPA, NRP, CFOD, SEMSO), Fire Chief, Upper Pine River Fire Protection District A massive leak of liquefied chlorine gas created a dangerous cloud over the city of Henderson, NV, in the early morning hours of May 6, 1991. Over 200 people (including firefighters) were examined at a local hospital for respiratory distress caused by inhalation of the chlorine and approximately 30 were admitted for treatment. Approximately 700 individuals were taken to shelters, and between 2,000 and 7,000 individuals were evacuated from the area. ASPR TRACIE interviewed Chief Bruce Evans (who was a firefighter- paramedic at the time of the incident), asking him to share his my first large-scale incident: the experiences and highlight how the PEPCON explosion. PEPCON was Access these U.S. Fire Administration Technical Reports fire and emergency response to a rocket fuel plant that supplied propellant used for the space for more information on these chemical incidents has changed incidents: over the years. shuttle program. This explosion sent a shock wave over the • Fire and Explosions at Rocket Corina Solé Brito, ASPR TRACIE entire Las Vegas Valley, blew out Fuel Plant (CSB): Can you please share windows that were miles away, • Massive Leak of Liquefied your experience in Henderson and created a small, multicolored Chlorine Gas and other related incidents, how mushroom cloud on the south end you think the field has changed of the valley. The Professional since then, and what you think Golfers’ Association (PGA) was the future holds? also in town, so there were satellite or injuries from flying debris were I am fortunate trucks and news trucks present. -

Fallout Model for the Robust Nuclear Earth Penetrator Blake Purnell the Radioactive Cloud Model
Fallout Model for the Robust Nuclear Earth Penetrator Blake Purnell Modeling radioactive fallout from nuclear explosions requires a description of the radioactive cloud and base surge and an atmospheric transport model for the cloud dispersion. The atmospheric transport model is independent of the radioactive nature of the dust and I will stick to a simple model in this study. The Radioactive Cloud Model Radioactive Release The first stage in understanding the fallout from a nuclear explosion is to estimate the amount of radioactivity released into the atmosphere. For external exposure to radiation, the main threat is from gamma-rays. The average gamma-ray activity1 produced in a nuclear explosion has been calculated as 530 megacuries per kiloton of fission yield at one hour after the explosion, with an average photon energy of 0.7 MeV. The data available on underground nuclear tests focuses on the fraction of the total activity found in “early” or “close-in” fallout ( Fc ), which measures only those particles that have been deposited in the first 24 2 hours . The fraction of the total activity released into the atmosphere ( f rel ) is greater than what appears in 3 the early fallout ( f rel > Fc ). The fraction Fc is dependant on the scaled depth of burst . A summary of the activity release data available for U.S. and Soviet underground tests are shown in Tables 1, Table 2, and Figure 1. Table 1: Activity Released from U.S. Underground Nuclear Tests. Test Yield Depth of Burst Scaled Depth of Burst Fraction of Total Activity 1/3 (kt) (m) (m/kt ) in Early Fallout (Fc) Jangle Sa 1.2 0 0 0.50 Jangle Ua 1.2 5.18 4.88 0.64 Teapot ESSa 1.2 20.4 19.2 0.46 Schoonerb 30 111 35.8 0.48 Cabrioletb 2.3 51.8 39.3 0.028 Buggyb,c 1.08 41.1 40.1 0.038 Sedanb,d 100 194 41.7 0.18 Danny Boya 0.43 33.5 44.4 0.04 Sulkyb 0.088 27.4 61.6 0.001 Neptunea 0.115 30.5 62.7 0.005 Blancaa 19 255 95.4 0.0005 a) Release fraction from Knox-65, Table 1.