VCOR Communications Policy V2.0 Updated By: R Brien & H Carruthers Updated: 1-DEC-2016 Page 2 of 14
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Psychiatry Attraction, Recruitment and Retention Needs Analysis Project
31 August 2017 Mr Trevor Hunt Manager, Mental Health and Drugs Workforce Portfolio Strategy and Reform Department of Health and Human Services 50 Lonsdale Street MELBOURNE VIC 3000 Dear Mr Hunt Re: Final report to DHHS on the Victorian Psychiatry Workforce The Victorian Branch of the Royal Australian and New Zealand College of Psychiatrists (RANZCP) wishes to present the Victorian Department of Health and Human Services (DHHS) with the report Psychiatry Attraction, Recruitment and Retention Needs Analysis Project. The DHHS approached the RANZCP in 2015 to undertake a project that would contribute to psychiatry workforce planning and development for Victoria’s public mental health sector. The project objectives, activities and deliverables were to: 1. Undertake a research and consultation exercise to identify factors and challenges influencing the psychiatry workforce. 2. Develop recommendations for future short-, medium- and long-term project work to address challenges faced by psychiatry in Victoria, with a particular focus on attraction, recruitment and retention to rural settings and to public mental health settings. 3. Produce a final report on methods, findings and proposals for possible future work that addresses attraction, recruitment and retention issues for psychiatry. The final report outlines the methods and findings from an extensive review of literature and data sources, together with more than 40 key informant interviews with psychiatrists working across Victoria. The report concludes that Victoria’s Approved Mental Health Services (AMHS) struggle to perform their role, with barely the capacity to care for Victorians during periods when they are severely mentally ill. Along with population and demographic changes over recent decades, public mental health services have experienced a reduction of funding in real terms to cope with these changes. -

Statement of Priorities
Statement of Priorities 2016-17 Agreement between Minister for Health and Monash Health To receive this publication in an accessible format phone 9096 1309, using the National Relay Service 13 36 77 if required, or email [email protected]. Authorised and published by the Victorian Government, 1 Treasury Place, Melbourne. © State of Victoria, Department of Health and Human Services, November 2016. ISBN/ISSN 2206-6403 Available at https://www2.health.vic.gov.au/hospitals-and-health-services/funding-performance- accountability/statement-of-priorities Contents Background ................................................................................................................................................. 4 Policy directions and priorities ................................................................................................................. 5 Government commitments ........................................................................................................................... 5 Part A: Strategic overview ......................................................................................................................... 8 Mission statement ......................................................................................................................................... 8 Service profile ............................................................................................................................................... 8 Strategic planning ...................................................................................................................................... -

HREC Web.Pdf
Hospital Name Principal Project No. Approving HREC Contact Phone Email Investigator HREC Person (for complaints or concerns) Alfred Hospital Dr Stephen Duffy 47/12 Alfred Hospital Ethics Ms Emily Bingle 03 9076 3619 [email protected] Committee Austin Hospital Dr David Clark H2012/04647 Austin Hospital HREC Ms Sianna 03 9496 5088 [email protected] Panagiotopoulos Ballarat Hospital Dr Ernesto Oqueli HREC/12/BHSSJOG/ Ballarat Health Dr Susan Joy Shea 03 5320 4787 [email protected] 63 Services HREC Bairnsdale Regional Dr Ka Chun Tse 2013-06 Latrobe Regional Patient Liaison Manager 03 5173 8003 [email protected] Health Services Hospital HREC Bendigo Health Dr Voltaire HREC/12/BHCG/23 Bendigo Healthcare HREC Chairperson 03 5454 6412 [email protected] Nadurata Group HREC (Sally McCarthy) Box Hill Hospital A/Prof Gishel New E09/1213 Eastern Health HREC HREC Chairperson 03 9895 3398 [email protected] Cabrini Dr Jeffrey 05-10-09-12 Cabrini HREC Anne Spence 03 9508 1376 [email protected] Lefkovits Epworth Healthcare Dr Ron Dick 55912 Epworth Healthcare HREC Coordinator 03 9426 8806 [email protected] Richmond HREC Epworth Healthcare Dr Ron Dick 55912 Epworth Healthcare HREC Coordinator 03 9426 8806 [email protected] Eastern HREC Frankston Hospital Dr Geoff Toogood HREC/12/PH/42 Peninsula Health HREC The Convenor 03 9788 1473 [email protected] Geelong Private Hospital A/Prof John CF12/1018- Monash HREC Executive Officer [email protected] Amerina 2012000481 03 9905 2052 Gippsland Base Hospital -

Annual Report 2019 Connected Care 15
Every patient matters and we are committed to providing high-quality patient care, delivered by a skilled, dedicated, compassionate workforce. We continue to innovate and find new ways of caring, in line with community need. Continually improving our patients’ experience, on each and every touchpoint of their journey with us, is always our goal. Epworth HealthCare is Victoria’s largest not-for-profit private hospital group, renowned for excellence in diagnosis, treatment, care and rehabilitation. Epworth is an innovator in Australia’s health system, embracing the latest in evidence-based medicine to pioneer treatments and services for our patients. Our vision: Caring for people. Innovating for a healthy community. Our purpose: Every patient matters. We strive to improve health outcomes and experience through compassion, collaboration, learning and innovation. Our values: Respect, excellence, community, compassion, integrity, accountability. Epworth was founded in 1920 as a 25-bed community intermediate hospital in Richmond by a Methodist minister for those on moderate incomes. We will celebrate our centenary next year. Today, our care is world-class, our technology is state- of-the-art and our ethos remains focused on our patients. This Annual Report details achievements and highlights of the 2018–19 financial year. It follows the pillars of our Strategic Plan: Connected Care, Empowered People, Innovative Practice and Sustainability. This report is available online at epworth.org.au We also highlight some of our patient stories - as patients are at the heart of all we do. Acknowledgement of Country Epworth HealthCare acknowledges the people of the Kulin Nations, on whose land we work and care for our patients. -

About Western Health
About Western Health Employing approximately 7,400 staff and more than 600 volunteers, Western Health has a strong philosophy of working with its local community to deliver excellence in patient care. Western Health has long-standing relationships with health providers in the western region of Melbourne and strong affiliations with numerous Colleges and academic institutions. Western Health manages three acute public hospitals: Western Hospital at Footscray; Sunshine Hospital at St Albans; and Williamstown Hospital. It also operates the Sunbury Day Hospital, and a Transition Care Program at Hazeldean in Williamstown. A wide range of community based services are also managed by Western Health, along with a large Drug and Alcohol Service. Services are provided to a population of approx. 879,000 people across the western region of Melbourne. Western Health provides a comprehensive, integrated range of services from its various sites; ranging from acute tertiary services in areas of emergency medicine, intensive care, medical and surgical services, through to subacute care and specialist ambulatory clinics. Western Health provides a combination of hospital and community-based services to aged, adult and paediatric patients and newborn babies. Our Partners We have long term partnerships with many providers across the region, including local government, primary and community care providers. This has culminated in the development and ongoing implementation of the Better Health Plan for the West. We are also partners in Strengthening Hospitals in Melbourne’s West – a coalition with our neighbouring health services, Djerriwarrh Health and Mercy health, which aims to build a cohesive view of how health services in the region can best meet the needs of our community now and into the future. -
Quality of Care Report
2015/16 QUALITY Account Communication and action 10 26 36 46 Person Centred Care Co-ordinated Care Right Care Safe Care Our Vision Together, caring for the West Our patients, staff, community and environment Our Purpose Leading the delivery of a connected and consistent patient experience and providing the best care to save and improve the lives of those in our community most in need. ACKNOWLEDGEMENT OF TRADITIONAL OWNERS: Western Health respectfully acknowledges the traditional owners of the land on which its sites stand as the Boon Wurrung and the Wurundjeri people of the greater Kulin Nation. 655 64 patients are cared surgical operations for overnight take place 560 369 patients see patients attend a doctor in an one of our three outpatient clinic emergency departments 333 patients are 400 discharged community providers partner with us to provide care 91 WHAT WE volunteers support staff and patients DO ON A 2306 and 6 students TYPICAL DAY meals are served from community engagement partnerships 43 patients are visited at home by our 150 Hospital in the patients require Home program interpreter services 393 15 patients are seen babies are welcomed by our Community into the world and Allied Health Services CONTENTS On a Typical Day at Western Health 01 Foreword 03 About Western Health 04 Best Care at Western Health 05 Accreditation 09 Person Centred Care 10 Co-ordinated Care 26 Right Care 36 Safe Care 46 Foreword This account on the quality of our care is a group of experienced clinicians examine adverse an important document for us at Western outcomes in a safe and supportive environment. -
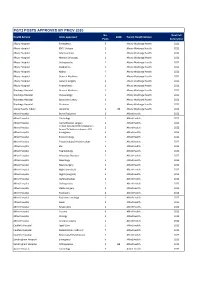
JMO POST DATA Current
PGY2 POSTS APPROVED BY PMCV 2020 No. Next Full Health Service Units approved 1008 Parent Health Service Posts Survey Due Albury Hospital Emergency 5 Albury Wodonga Health 2021 Albury Hospital ENT/ Urology 1 Albury Wodonga Health 2021 Albury Hospital Intensive Care 1 Albury Wodonga Health 2021 Albury Hospital Medical Oncology 1 Albury Wodonga Health 2021 Albury Hospital Orthopaedics 1 Albury Wodonga Health 2021 Albury Hospital Paediatrics 1 Albury Wodonga Health 2021 Albury Hospital Nights 4 Albury Wodonga Health 2021 Albury Hospital General Medicine 1 Albury Wodonga Health 2021 Albury Hospital General Surgery 2 Albury Wodonga Health 2021 Albury Hospital Anaesthetics 1 Albury Wodonga Health 2021 Wodonga Hospital General Medicine 1 Albury Wodonga Health 2021 Wodonga Hospital Gynaecology 1 Albury Wodonga Health 2021 Wodonga Hospital Specialist Surgery 1 Albury Wodonga Health 2021 Wodonga Hospital Geriatrics 1 Albury Wodonga Health 2021 Mercy Health, Albury Geriatrics 1 23 Albury Wodonga Health 2021 Alfred Hospital Burns/Faciomax 2 Alfred Health 2022 Alfred Hospital Cardiology 2 Alfred Health 2022 Alfred Hospital Cardiothoracic surgery 2 Alfred Health 2022 Critical Care (2x3mth rotations in Alfred Hospital 2 Alfred Health 2022 Anaes/ 2x3mth rotations in ICU) Alfred Hospital Emergency 4 Alfred Health 2022 Alfred Hospital Endocrinology 1 Alfred Health 2022 Alfred Hospital Endocrinology/ rheumatology 1 Alfred Health 2022 Alfred Hospital ENT 1 Alfred Health 2022 Alfred Hospital Haematology 1 Alfred Health 2022 Alfred Hospital Infectious Diseases -

The Royal Childrens Hospital
Statement of Priorities 2019-20 Agreement between the Minister for Health and the Royal Children’s Hospital. To receive this publication in an accessible format phone 9096 1309, using the National Relay Service 13 36 77 if required, or email [email protected]. Authorised and published by the Victorian Government, 1 Treasury Place, Melbourne. © State of Victoria, Department of Health and Human Services, November 2019. ISSN 2206-6462 Available at https://www2.health.vic.gov.au/hospitals-and-health-services/funding-performance- accountability/statement-of-priorities The Royal Children’s Hospital Statement of Priorities 2019–20 ii Contents Contents ........................................................................................................................................ iii Background .................................................................................................................................... 4 Strategic priorities ......................................................................................................................... 5 Government commitments .............................................................................................................. 5 Part A: Strategic overview ............................................................................................................ 6 Mission statement ............................................................................................................................ 6 Service profile ................................................................................................................................. -

Research Report 2016 Report | Research Health Western 1 Foreword
2016 Research Report Our Vision Our Values Together, caring for the West, Compassion our patients, staff, community Consistently acting with empathy and integrity and environment Accountability Taking responsibility for our decisions and actions Respect Respect for the rights, beliefs and choice of every individual Excellence Inspiring and motivating innovation and achievement Safety Prioritising safety as an essential part of everyday practice Our Purpose Our Strategic Aims Leading the delivery of a Growing and improving the delivery of safe, high quality care connected and consistent patient Connecting the care provided to our community experience and providing the best care to save and improve Communicating with our patients, our partners and each the lives of those in our other with transparency and purpose community most in need Being socially responsible and using resources sustainably Valuing and empowering our people Acknowledgement of Traditional Owners Western Health respectfully acknowledges the traditional owners of the land on which its sites stand as the Boon Wurrung and the Wurundjeri people of the greater Kulin Nation. Contents Foreword 2 Melbourne Neuropsychiatry Centre 58 Research 2016 3 Nephrology 62 Research Directorate 4 Neurology 66 Office for Research 8 Nursing and Midwifery 68 Western Health Research Grants 9 Oncology and Haematology 74 Research Week: Awards and Prizes 10 Paediatric Emergency Medicine 80 Allied Health and Community Services 12 Pharmacy 82 Nutrition 14 Respiratory and Sleep Disorders 84 Occupational -

Postgraduate Medical Council of Victoria Inc. Health
POSTGRADUATE MEDICAL COUNCIL OF VICTORIA INC. CONTENTS Albury Wodonga Health .......................................................................................................................... 5 Alexandra District Health / Eastern Health Collaborative ...................................................................... 5 Alfred Health ........................................................................................................................................... 6 Austin Health........................................................................................................................................... 6 Austin Health – COMBINED NURSING / MENTAL HEALTH .................................................................... 7 Bairnsdale Regional Health Service......................................................................................................... 7 Ballarat Health Services .......................................................................................................................... 8 Ballarat Health Services – Care of the Older Person (COOPS) ................................................................ 8 Barwon Health ........................................................................................................................................ 9 Barwon Health & Great Ocean Road Health Collaborative .................................................................... 9 Bass Coast Health ................................................................................................................................. -

About Western Health Our Focus: Best Care Our Vision Our Purpose Our
About Western Health Western Health provides services to a region with more than 800,000 people, taking in some of the fastest growing suburbs in Australia. Our patients and their families speak more than 110 different languages. The rapid population growth and extraordinary diversity of our service region is reflected in the scale of demand for our services, delivered through Footscray, Sunshine and Williamstown Hospitals and Sunbury Day Hospital, as well as through Hazeldean Transition Care; dozens of community clinic settings; as well as in patients’ homes. Over the past 12 months, our 6,100 staff provided care to patients through 184,000 medical outpatient appointments, 128,000 emergency department presentations and more than 110,000 inpatient separations and were capably supported by close to 600 volunteers. In 2014, Western Health was named the Premier’s Health Service of the Year – a prestigious accolade recognising leadership and excellence in the provision of publicly-funded healthcare for the Victorian community. Our Focus: Best Care At Western Health we are committed to high quality, safe and person centred patient care. The Western Health “Best Care” Framework for Quality, Safety and the Patient Experience outlines how Western Health – in partnership with our patients and their families; building on the strengths of our clinical and health support staff; and backed by managers, the Executive and the Board – will continue to strive for our vision of Best Care. Our Vision Together, caring for the West, our patients, staff, community and environment. Our Purpose Working collaboratively to provide quality health and well-being services for the people of the West. -

Professor John Olver Victor Smorgon Chair of Rehabilitation Medicine at Monash University
Issue 170 Winter 2009 Excellence. Everywhere. Everyday. Professor John Olver Victor Smorgon Chair of Rehabilitation Medicine at Monash University John Olver, the face of Epworth Rehabilitation for almost 30 years, has recently been appointed to the Victor Smorgon Chair of Rehabilitation Medicine at Monash University. His new position comprises dual relationships with Epworth HealthCare and Monash University incorporating Epworth Rehabilitation and the Faculty of Medicine, Nursing and Health Sciences. It is the first academic Chair in the “His role over the next five years the academic aspects of rehabilitative Faculty of Medicine at Epworth is to foster excellence in research, medicine; giving students a better HealthCare and only the second time policy development and professional understanding of the topic; and that a Victorian University has selected activities while ensuring that all extending the role of Monash in the a Chair in partnership with a private rehabilitation medicine service Victorian health system. hospital group. commitments to patient care, “This is a new and very exciting teaching and research are maintained. Philip Williams, President of Epworth’s relationship that will foster Mr Williams said. Board of Management, is delighted improvements to clinical research with the news, noting that it is Professor Olver said he looks forward and to the care that doctors and the first in a series of professorial to working in partnership with allied health professionals can offer appointments at Epworth. Professor Steve Wesselingh, Dean patients undergoing rehabilitation. of the Faculty of Medicine, Nursing We look forward to building on “John’s appointment reflects our and Health Sciences at Monash, and these ties with Epworth HealthCare,” commitment to post-graduate Epworth’s Group CEO Alan Kinkade, Professor Wesselingh said.