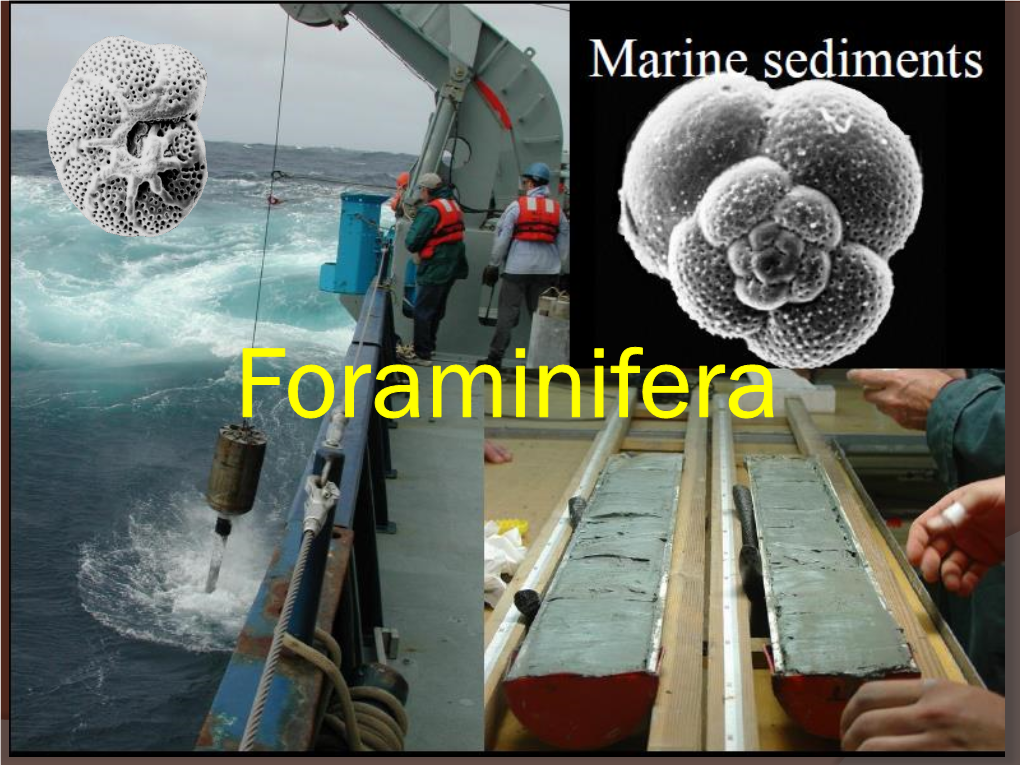
Planktic and Smaller Benthic Foraminifera Are Prepared As Follows: • Crush the Bulk Rock Into Roughly 5-Mm Fragments
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Parasitology Protozoan Parasites and Their Molecules Molecular Parasitology Julia Walochnik • Michael Duchêne Editors
Julia Walochnik Michael Duchêne Editors Molecular Parasitology Protozoan Parasites and their Molecules Molecular Parasitology Julia Walochnik • Michael Duchêne Editors Molecular Parasitology Protozoan Parasites and their Molecules Editors Julia Walochnik Michael Duchêne Institute of Specifi c Prophylaxis Institute of Specifi c Prophylaxis and Tropical Medicine and Tropical Medicine Center for Pathophysiology, Infectiology Center for Pathophysiology, Infectiology and Immunology and Immunology Medical University of Vienna Medical University of Vienna Vienna Vienna Austria Austria ISBN 978-3-7091-1415-5 ISBN 978-3-7091-1416-2 (eBook) DOI 10.1007/978-3-7091-1416-2 Library of Congress Control Number: 2016947730 © Springer-Verlag Wien 2016 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifi cally the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfi lms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specifi c statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. The publisher, the authors and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. Neither the publisher nor the authors or the editors give a warranty, express or implied, with respect to the material contained herein or for any errors or omissions that may have been made. -

Paramoeba Pemaquidensis (Sarcomastigophora: Paramoebidae) Infestation of the Gills of Coho Salmon Oncorhynchus Kisutch Reared in Sea Water
Vol. 5: 163-169, 1988 DISEASES OF AQUATIC ORGANISMS Published December 2 Dis. aquat. Org. Paramoeba pemaquidensis (Sarcomastigophora: Paramoebidae) infestation of the gills of coho salmon Oncorhynchus kisutch reared in sea water Michael L. ~ent'l*,T. K. Sawyer2,R. P. ~edrick~ 'Battelle Marine Research Laboratory, 439 West Sequim Bay Rd, Sequim, Washington 98382, USA '~esconAssociates, Inc., Box 206, Turtle Cove, Royal Oak, Maryland 21662, USA 3~epartmentof Medicine, School of Veterinary Medicine, University of California, Davis, California 95616, USA ABSTRACT: Gill disease associated with Paramoeba pemaquidensis Page 1970 (Sarcomastigophora: Paramoebidae) infestations was observed in coho salmon Oncorhynchus lasutch reared in sea water Fish reared in net pens in Washington and in land-based tanks in California were affected. Approxi- mately 25 O/O mortality was observed in the net pens in 1985, and the disease recurred in 1986 and 1987. Amoeba infesting the gill surfaces elicited prominent epithelia1 hyperplasia. Typical of Paramoeba spp., the parasite had a Feulgen positive parasome (Nebenkorper) adjacent to the nucleus and floatlng and transitional forms had digitiform pseudopodia. We have established cultures of the organism from coho gills; it grows rapidly on Malt-yeast extract sea water medium supplemented with Klebsiella bacteria. Ultrastructural characteristics and nuclear, parasome and overall size of the organism in study indicated it is most closely related to the free-living paramoeba P. pemaquidensis. The plasmalemma of the amoeba from coho gills has surface filaments. Measurements (in pm) of the amoeba under various conditions are as follows: transitional forms directly from gills 28 (24 to 30),locomotive forms from liquid culture 21 X 17 (15 to 35 X 11 to 25), and locomotive forms from agar culture 25 X 20 (15 to 38 X 15 to 25). -

Ebriid Phylogeny and the Expansion of the Cercozoa
ARTICLE IN PRESS Protist, Vol. 157, 279—290, May 2006 http://www.elsevier.de/protis Published online date 26 May 2006 ORIGINAL PAPER Ebriid Phylogeny and the Expansion of the Cercozoa Mona Hoppenrath1, and Brian S. Leander Canadian Institute for Advanced Research, Program in Evolutionary Biology, Departments of Botany and Zoology, University of British Columbia, Vancouver, BC, Canada, V6T 1Z4 Submitted November 30, 2005; Accepted March 4, 2006 Monitoring Editor: Herve´ Philippe Ebria tripartita is a phagotrophic flagellate present in marine coastal plankton communities worldwide. This is one of two (possibly four) described extant species in the Ebridea, an enigmatic group of eukaryotes with an unclear phylogenetic position. Ebriids have never been cultured, are usually encountered in low abundance and have a peculiar combination of ultrastructural characters including a large nucleus with permanently condensed chromosomes and an internal skeleton composed of siliceous rods. Consequently, the taxonomic history of the group has been tumultuous and has included a variety of affiliations, such as silicoflagellates, dinoflagellates, ‘radiolarians’ and ‘neomonads’. Today, the Ebridea is treated as a eukaryotic taxon incertae sedis because no morphological or molecular features have been recognized that definitively relate ebriids with any other eukaryotic lineage. We conducted phylogenetic analyses of small subunit rDNA sequences from two multi-specimen isolations of Ebria tripartita. The closest relatives to the sequences from Ebria tripartita are environmental sequences from a submarine caldera floor. This newly recognized Ebria clade was most closely related to sequences from described species of Cryothecomonas and Protaspis. These molecular phylogenetic relationships were consistent with current ultrastructural data from all three genera, leading to a robust placement of ebriids within the Cercozoa. -

Phylum Sarcomastigophora
MINISTRY OF THE PUBLIC HEALTH OF UKRAINE ZAPORIZHZHIA STATE MEDICAL UNIVERSITY DEPARTMENT OF MEDICAL BIOLOGY A.B. Prikhodko, A.P. Popovich, T.I. Yemets, A.Y. Maleeva POPULATION-SPECIES, BIOGEOCENOTIC AND BIOSPHERICAL LEVELS OF LIVING THINGS ORGANIZATION MODULE II TEXT-BOOK for the first year training students of the medical faculty Zaporizhzhia 2017 Ratified on meeting of the Central methodical committee of ZSMU and it is recommended for the use in educational process for foreign students. (protocol N 3 from 02.03.2017) Authors: A. B. Prikhodko, A. P. Popovich, T. I. Yemets, A. Y. Maleeva Reviewers: E. V. Alexandrova, Head of Biological Chemistry and Laboratory Diagnosis Department Zaporozhye State Medical University, Doctor of Chemical Sciences; A. V. Abramov, Doctor of Medical Sciences, Professor of Department Pathological Physiology Population-species, biogeocenotic and biospherical levels of living things organization. Module II : text-book for the first year training students of the medical faculty / comp. : A. B. Prikhodko, A. P. Popovich, T. I. Yemets, A. Y. Maleeva. – Zaporizhzhia : ZSMU, 2017. – 108 p. Medical Parasitology is a fundamental discipline within the medical sciences. Study of the structure, organization and life-cycles of different parasites gives the doctors strong knowledge about parasitizm for searching the most effective methods of treatment. The present Text Book for the first year training students of the Medical faculty has been written in accordance with the Academic Curriculum on Medical Biology accepted by all Medical University of Ukraine. Efforts have been made to provide latest material facts. Improved illustration wherever necessary are provided, for a better understanding of the subject by the students. -

Molecular Characterisation of Neoparamoeba Strains Isolated from Gills of Scophthalmus Maximus
DISEASES OF AQUATIC ORGANISMS Vol. 55: 11–16, 2003 Published June 20 Dis Aquat Org Molecular characterisation of Neoparamoeba strains isolated from gills of Scophthalmus maximus Ivan Fiala1, 2, Iva Dyková1, 2,* 1Institute of Parasitology, Academy of Sciences of the Czech Republic and 2Faculty of Biological Sciences, University of South Bohemia, Brani$ovská 31, 370 05 >eské Budeˇ jovice, Czech Republic ABSTRACT: Small subunit ribosomal RNA gene sequences were determined for 5 amoeba strains of the genus Neoparamoeba Page, 1987 that were isolated from gills of Scophthalmus maximus (Lin- naeus, 1758). Phylogenetic analyses revealed that 2 of 5 morphologically indistinguishable strains clustered with 6 strains identified previously as N. pemaquidensis (Page, 1970). Three strains branched as a clade separated from N. pemaquidenis and N. aestuarina (Page, 1970) clades. Our analyses suggest that these 3 strains could be representatives of an independent species. In a more comprehensive eukaryotic tree, strains belonging to Neoparamoeba spp. formed a monophyletic group with a sister-group relationship to Vannella anglica Page, 1980. They did not cluster with Gymnamoebae of the families Hartmannellidae, Flabellulidae, Leptomyxidae or Amoebidae presently available in GenBank. KEY WORDS: Paramoeba · Neoparamoeba · SSU rDNA · Phylogenetic position Resale or republication not permitted without written consent of the publisher INTRODUCTION Sequences of the SSU rRNA gene were made accessi- ble in GenBank in May 2002. Amoebic gill disease (AGD), repeatedly declared As a first step, aimed at unravelling the biology and one of the most serious diseases affecting farmed taxonomy of the agent of AGD in turbot Scophthalmus salmonids Salmo salar Linnaeus, 1758 and Oncorhyn- maximus, comparative light and transmission electron chus mykiss (Walbaum, 1792) in the last 2 decades microscopical studies of 6 Neoparamoeba strains indi- (Kent et al. -

Neoparamoeba Sp. and Other Protozoans on the Gills of Atlantic Salmon Salmo Salar Smolts in Seawater
DISEASES OF AQUATIC ORGANISMS Vol. 76: 231–240, 2007 Published July 16 Dis Aquat Org Neoparamoeba sp. and other protozoans on the gills of Atlantic salmon Salmo salar smolts in seawater Mairéad L. Bermingham*, Máire F. Mulcahy Environmental Research Institute, Aquaculture and Fisheries Development Centre, Department of Zoology, Ecology and Plant Science, National University of Ireland, Cork, Ireland ABSTRACT: Protozoan isolates from the gills of marine-reared Atlantic salmon Salmo salar smolts were cultured, cloned and 8 dominant isolates were studied in detail. The light and electron-micro- scopical characters of these isolates were examined, and 7 were identified to the generic level. Struc- ture, ultrastructure, a species-specific immunofluorescent antibody test (IFAT), and PCR verified the identity of the Neoparamoeba sp. isolate. Five other genera of amoebae, comprising Platyamoeba, Mayorella, Vexillifera, Flabellula, and Nolandella, a scuticociliate of the genus Paranophrys, and a trypanosomatid (tranosomatid-bodonid incertae sedis) accompanied Neoparamoeba sp. in the gills. The pathogenic potential of the isolated organisms, occurring in conjunction with Neoparamoeba sp. in the gills of cultured Atlantic salmon smolts in Ireland, remains to be investigated KEY WORDS: Amoebic gill disease · Neoparamoeba sp. · Amoebae · Platyamoeba sp. · Scuticociliates · Trypanosomatids Resale or republication not permitted without written consent of the publisher INTRODUCTION 1990, Palmer et al. 1997). However, simultaneous iso- lation of amoebae other than Neoparamoeba sp. from Various protozoans have been associated with gill the gills of clinically diseased fish has raised the disease in fish. Those causing the most serious mor- question of the possible involvement of such amoe- talities in fish are generally free-living species of bae in the disease (Dyková et al. -

Diversity, Phylogeny and Phylogeography of Free-Living Amoebae
School of Doctoral Studies in Biological Sciences University of South Bohemia in České Budějovice Faculty of Science Diversity, phylogeny and phylogeography of free-living amoebae Ph.D. Thesis RNDr. Tomáš Tyml Supervisor: Mgr. Martin Kostka, Ph.D. Department of Parasitology, Faculty of Science, University of South Bohemia in České Budějovice Specialist adviser: Prof. MVDr. Iva Dyková, Dr.Sc. Department of Botany and Zoology, Faculty of Science, Masaryk University České Budějovice 2016 This thesis should be cited as: Tyml, T. 2016. Diversity, phylogeny and phylogeography of free living amoebae. Ph.D. Thesis Series, No. 13. University of South Bohemia, Faculty of Science, School of Doctoral Studies in Biological Sciences, České Budějovice, Czech Republic, 135 pp. Annotation This thesis consists of seven published papers on free-living amoebae (FLA), members of Amoebozoa, Excavata: Heterolobosea, and Cercozoa, and covers three main topics: (i) FLA as potential fish pathogens, (ii) diversity and phylogeography of FLA, and (iii) FLA as hosts of prokaryotic organisms. Diverse methodological approaches were used including culture-dependent techniques for isolation and identification of free-living amoebae, molecular phylogenetics, fluorescent in situ hybridization, and transmission electron microscopy. Declaration [in Czech] Prohlašuji, že svoji disertační práci jsem vypracoval samostatně pouze s použitím pramenů a literatury uvedených v seznamu citované literatury. Prohlašuji, že v souladu s § 47b zákona č. 111/1998 Sb. v platném znění souhlasím se zveřejněním své disertační práce, a to v úpravě vzniklé vypuštěním vyznačených částí archivovaných Přírodovědeckou fakultou elektronickou cestou ve veřejně přístupné části databáze STAG provozované Jihočeskou univerzitou v Českých Budějovicích na jejích internetových stránkách, a to se zachováním mého autorského práva k odevzdanému textu této kvalifikační práce. -

Protista (PDF)
1 = Astasiopsis distortum (Dujardin,1841) Bütschli,1885 South Scandinavian Marine Protoctista ? Dingensia Patterson & Zölffel,1992, in Patterson & Larsen (™ Heteromita angusta Dujardin,1841) Provisional Check-list compiled at the Tjärnö Marine Biological * Taxon incertae sedis. Very similar to Cryptaulax Skuja Laboratory by: Dinomonas Kent,1880 TJÄRNÖLAB. / Hans G. Hansson - 1991-07 - 1997-04-02 * Taxon incertae sedis. Species found in South Scandinavia, as well as from neighbouring areas, chiefly the British Isles, have been considered, as some of them may show to have a slightly more northern distribution, than what is known today. However, species with a typical Lusitanian distribution, with their northern Diphylleia Massart,1920 distribution limit around France or Southern British Isles, have as a rule been omitted here, albeit a few species with probable norhern limits around * Marine? Incertae sedis. the British Isles are listed here until distribution patterns are better known. The compiler would be very grateful for every correction of presumptive lapses and omittances an initiated reader could make. Diplocalium Grassé & Deflandre,1952 (™ Bicosoeca inopinatum ??,1???) * Marine? Incertae sedis. Denotations: (™) = Genotype @ = Associated to * = General note Diplomita Fromentel,1874 (™ Diplomita insignis Fromentel,1874) P.S. This list is a very unfinished manuscript. Chiefly flagellated organisms have yet been considered. This * Marine? Incertae sedis. provisional PDF-file is so far only published as an Intranet file within TMBL:s domain. Diplonema Griessmann,1913, non Berendt,1845 (Diptera), nec Greene,1857 (Coel.) = Isonema ??,1???, non Meek & Worthen,1865 (Mollusca), nec Maas,1909 (Coel.) PROTOCTISTA = Flagellamonas Skvortzow,19?? = Lackeymonas Skvortzow,19?? = Lowymonas Skvortzow,19?? = Milaneziamonas Skvortzow,19?? = Spira Skvortzow,19?? = Teixeiromonas Skvortzow,19?? = PROTISTA = Kolbeana Skvortzow,19?? * Genus incertae sedis. -

PHYLUM APICOMPLEXA (Levine 1970) Clase Sporozoa
Reino Protista, subreino Protozoa › Unicelulares › Eucariotas › Reproducción asexuada o sexuada › Movilidad variable › Mayoría tienen nutrición de tipo heterótrofa › Pueden vivir libremente o actuar como parásitos. Con membrana nuclear Con nucleolo Membrana celular con HC y esteroles Con citoesqueleto Con organelos: mitocondrias, RE, Golgi, vacuolas y lisosomas Capaces de realizar endocitosis y exocitosis Con ribosomas compuestos por subunidades 40S y 60S NÚCLEO › CROMATINA central y periférica › NUCLEOLOS › MEMBRANA NUCLEAR CITOPLASMA › VACUOLAS › RE, RIBOSOMAS, GOLGI › MITOCONDRIAS › LISOSOMAS (contienen proteasas) › ELEMENTOS DE SOSTÉN (axostilo) › WFB (cuerpos formadores de pared) › MEMBRANA PLASMÁTICA simple o rodeada de glicocálix TROFOZOÍTO: forma activa QUISTE: forma de › resistencia › Transmisión › Multiplicación OOQUISTE: › correspondiente a la etapa de reproducción sexuada. › en algunas especies. ASEXUADA › FISIÓN BINARIA › DIVISIÓN MÚLTIPLE › BROTAMIENTO EXTERNO o plasmotomía › ENDODIOGENIA: formación de 2 células hijas por brotamiento interno › ESQUIZOGONIA: división del núcleo celular en gran número de núcleos secundarios que se rodean de citoplasma. MEROGONIA: produce merozoítos y esquizontes ESPOROGONIA: produce esporozoítos (dentro del cigoto) SEXUADA › GAMETOGONIA: produce gametos y un cigoto SEUDÓPODOS: › LOBÓPODOS: ej. E. histolytica › FILÓPODOS: ej. Acanthamoeba FLAGELOS CILIAS COMPLEJO APICAL REINO PROTISTA › SUB REINO PROTOZOA PHYLUM SARCOMASTIGOPHORA Sub Phylum Mastigophora Sub Phylum Sarcodina -

The Protists
The Protists (160,000 living & extinct species; estimates to 200,000 actual species) mostly single-celled, eukaryotes restricted to aquatic, or moist environments: oceans, ponds, lakes, rivers, damp soil, tree bark, snow, etc.; some species are colonial or multicellular; autotrophs & heterotrophs; with or without cell walls; most motile. Genetic analyses have dramatically changed the classification scheme if this group of organisms. In this course we will discuss examples of three main types of protists; algae, protozoa and slime & water molds. Algae [Plant-Like Protists] (22,000 living species) diverse group of mostly unicellular protists, some colonial or multicellular; restricted to aquatic or moist environments: oceans, ponds, lakes, rivers, soil, bark, snow, etc.; photosynthetic with chloroplasts containing chlorophyll and other pigments, most motile; most with cell wall; cell walls of cellulose, proteins,, silica or other materials classified according to their kinds of photosynthetic pigments and composition of cell wall Fire Algae (Dinoflagellates; Phylum Pyrrhophyta, 2100 species) unicellular, many symbiotic as zooxanthellae; some produce cell walls of armored plates, blooms produce toxic red tides, bioluminescent Diatoms (Glass Algae; Phylum Chrysophyta, 28000 living & extinct species) most abundant group of algae; unicellular, radial symmetry, cell walls contain silica; common in freshwater and oceans; source of diatomaceous earth; gliding movement, Euglenas (Phylum Euglenophyta, 1000 species) unicellular, only algae without -

Chapter 12: Fungi, Algae, Protozoa, and Parasites
I. FUNGI (Mycology) u Diverse group of heterotrophs. u Many are ecologically important saprophytes(consume dead and decaying matter) Chapter 12: u Others are parasites. Fungi, Algae, Protozoa, and u Most are multicellular, but yeasts are unicellular. u Most are aerobes or facultative anaerobes. Parasites u Cell walls are made up of chitin (polysaccharide). u Over 100,000 fungal species identified. Only about 100 are human or animal pathogens. u Most human fungal infections are nosocomial and/or occur in immunocompromised individuals (opportunistic infections). u Fungal diseases in plants cause over 1 billion dollars/year in losses. CHARACTERISTICS OFFUNGI (Continued) CHARACTERISTICS OFFUNGI 2. Molds and Fleshy Fungi 1. Yeasts u Multicellular, filamentous fungi. u Unicellular fungi, nonfilamentous, typically oval or u Identified by physical appearance, colony characteristics, spherical cells. Reproduce by mitosis: and reproductive spores. u Fission yeasts: Divide evenly to produce two new cells u Thallus: Body of a mold or fleshy fungus. Consists of many (Schizosaccharomyces). hyphae. u Budding yeasts: Divide unevenly by budding (Saccharomyces). u Hyphae (Sing: Hypha): Long filaments of cells joined together. Budding yeasts can form pseudohypha, a short chain of u Septate hyphae: Cells are divided by cross-walls (septa). undetached cells. u Coenocytic (Aseptate) hyphae: Long, continuous cells that are not divided by septa. Candida albicans invade tissues through pseudohyphae. Hyphae grow by elongating at the tips. u Yeasts are facultative anaerobes, which allows them to Each part of a hypha is capable of growth. grow in a variety of environments. u Vegetative Hypha: Portion that obtains nutrients. u Reproductive or Aerial Hypha: Portion connected with u When oxygen is available, they carry out aerobic respiration. -

CHAPTER 11 PROTOZOANS Protozoa Are a Diverse Assemblage with Mixed Affinities. A. They Lack a Cell Wall. B. They Have At
CHAPTER 11 PROTOZOANS Protozoa are a diverse assemblage with mixed affinities. a. They lack a cell wall. b. They have at least one motile stage in the life cycle. c. Most ingest their food. Biological Contributions 1. Protozoa have intracellular specialization or organization of organelles in cells. 2. Cells may have distinct functions; some colonial protozoa have separate somatic and reproductive zooids. 3. Asexual reproduction occurs by mitotic division. 4. Some have true sexual reproduction with zygote formation. 5. Responses to stimuli represent the simplest reflexes and inborn behaviors known. 6. Shelled protozoa have the simplest exoskeletons. 7. Basic enzymes systems support all types of nutrition: autotrophic, saprozoic and holozoic. 8. Many have developed means of locomotion General Features 1. A protozoan is a complete organism in which all life activities are carried on within the limits of a single plasma membrane. 2. Phylogenetic studies show that protozoa do not form a monophyletic group. 3. Over 64,000 species are named; half are fossils. 4. Although they are unicellular organisms, protozoan cell organelles are highly specialized. 5. They are ecological diverse, widely dispersed, but many are limited to narrow environmental ranges. 6. They can be fantastically numerous, forming gigantic ocean soil deposits. 7. About 10,000 are symbiotic in or on animals or plants; some are human disease agents. 8. Some are colonial with multicellular stages but have noncolonial forms. 9. Protozoa have only one non-reproductive cell type and lack embryonic development; embryonic development is one of the criteria for metazoa. Characteristics of Protozoan Phyla 1. They are unicellular with some colonial and multicellular stages.