DISEASES of the ENDOCRINE SYSTEM Lectures from Endocrinology
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
2 Surgical Anatomy
2 Surgical Anatomy Nancy Dugal Perrier, Michael Sean Boger contents 2.2 Morphology 2.1 Introduction . 7 The paired retroperitoneal adrenal glands are found 2.2 Morphology ...7 in the middle of the abdominal cavity, residing on 2.3 Relationship of the Adrenal Glands to the Kidneys ...10 the superior medial aspect of the upper pole of each 2.4 Blood Supply, Innervation, and Lymphatics ...10 kidney (Fig. 1). However, this location may vary 2.4.1 Arterial . 10 depending on the depth of adipose tissue.By means of 2.4.2 Venous ...10 pararenal fat and perirenal fascia,the adrenals contact 2.4.3 Innervation ...11 the superior portion of the abdominal wall. These 2.4.4 Lymphatic . 11 structures separate the adrenals from the pleural re- 2.5 Left Adrenal Gland Relationships ...11 flection, ribs, and the subcostal, sacrospinalis, and 2.6 Right Adrenal Gland Relationships ...14 latissimus dorsi muscles [2].Posteriorly,the glands lie 2.7 Summary ...17 References . 17 near the diaphragmatic crus and arcuate ligament [10]. Laterally, the right adrenal resides in front of the 12th rib and the left gland is in front of the 11th and 12th ribs [2]. Each adrenal gland weighs approximately 2.1 Introduction Liver Adrenal gland The small paired adrenal glands have a grand history. Eustachius published the first anatomical drawings of the adrenal glands in the mid-sixteenth century [17].In 1586, Piccolomineus and Baunin named them the suprarenal glands. Nearly two-and-a-half centuries later, Cuvier described the anatomical division of each gland into the cortex and medulla. -

Endocrinology Resident Profile Jill Trinacty
Endocrinology Resident Profile Jill Trinacty July 2017 About me My name is Jill Trinacty, and I was raised in Berwick, Nova Scotia. I went completed my BSc. (Honours) at Saint Francis Xavier University in Antigonish, Nova Scotia. I spent a year as the Active Living Coordinator with the Town of Kentville then completed my MD at Dalhousie University. I moved to Ottawa, Ontario in 2013 for my residency in Internal Medicine at the University of Ottawa and am currently a PGY-5 in Endocrinology and Metabolism. Why I chose General Endocrinology In medical school I became interested in almost every area of medicine, but ultimately applied to internal medicine for a number of reasons. I have always been interested in Endocrinology, specifically diabetes. Diabetes affects so many people in our communities and can have significant morbidity and mortality. I liked the detail of internal medicine and the complexity of patients. Having some previous experience in public health, I knew that I wanted a career that would allow me to discuss lifestyle changes as an aspect of therapy – specifically nutrition and physical activity. I also wanted a career that would allow for work/life balance. Seeing the day-to-day lifestyle of each internal medicine subspecialty confirmed that Endocrinology was the right fit for me. Clinical Life What does a typical day of clinical duties involve? This is an example of my typical daily and weekly schedule: Endocrinology – A typical day 7:30 – 8:00 Clerical work / Chief resident duties. Review emails, follow up on patient results, approve vacation requests, make call schedule. -

Anatomical Variations in the Arterial Supply of the Suprarenal Gland. Int J Health Sci Res
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Anatomical Variations in the Arterial Supply of the Suprarenal Gland Sushma R.K1, Mahesh Dhoot2, Hemant Ashish Harode2, Antony Sylvan D’Souza3, Mamatha H4 1Lecturer, 2Postgraduate, 3Professor & Head, 4Assistant Professor; Department of Anatomy, Kasturba Medical College, Manipal University, Manipal-576104, Karnataka, India. Corresponding Author: Mamatha H Received: 29/03//2014 Revised: 17/04/2014 Accepted: 21/04/2014 ABSTRACT Introduction: Suprarenal gland is normally supplied by superior, middle and inferior suprarenal arteries which are the branches of inferior phrenic, abdominal aorta and renal artery respectively. However the arterial supply of the suprarenal gland may show variations. Therefore a study was conducted to find the variations in the arterial supply of Suprarenal Gland. Materials and methods: 20 Formalin fixed cadavers, were dissected bilaterally in the department of Anatomy, Kasturba Medical College, Manipal to study the arterial supply of the suprarenal gland, which were photographed and different variations were noted. Results: Out of 20 cadavers variations were observed in five cases in the arterial pattern of supra renal gland. We found that in one cadaver superior supra renal artery on the left side was arising directly from the coeliac trunk. Another variation was observed on the right side ina cadaver that inferior and middle suprarenal arteries were arising from accessory renal artery and on the right side it gave another small branch to the gland. Conclusion: Variations in the arterial pattern of suprarenal gland are significant for radiological and surgical interventions. KEY WORDS: Suprarenal gland, suprarenal artery, renal artery, abdominal aorta, inferior phrenic artery INTRODUCTION accessory renal arteries (ARA). -

A Case of Malignant Insulinoma Responsive to Somatostatin Analogs
Caliri et al. BMC Endocrine Disorders (2018) 18:98 https://doi.org/10.1186/s12902-018-0325-4 CASE REPORT Open Access A case of malignant insulinoma responsive to somatostatin analogs treatment Mariasmeralda Caliri1†, Valentina Verdiani1†, Edoardo Mannucci2, Vittorio Briganti3, Luca Landoni4, Alessandro Esposito4, Giulia Burato5, Carlo Maria Rotella2, Massimo Mannelli1 and Alessandro Peri1* Abstract Background: Insulinoma is a rare tumour representing 1–2% of all pancreatic neoplasms and it is malignant in only 10% of cases. Locoregional invasion or metastases define malignancy, whereas the dimension (> 2 cm), CK19 status, the tumor staging and grading (Ki67 > 2%), and the age of onset (> 50 years) can be considered elements of suspect. Case presentation: We describe the case of a 68-year-old man presenting symptoms compatible with hypoglycemia. The symptoms regressed with food intake. These episodes initially occurred during physical activity, later also during fasting. The fasting test was performed and the laboratory results showed endogenous hyperinsulinemia compatible with insulinoma. The patient appeared responsive to somatostatin analogs and so he was treated with short acting octreotide, obtaining a good control of glycemia. Imaging investigations showed the presence of a lesion of the uncinate pancreatic process of about 4 cm with a high sst2 receptor density. The patient underwent exploratory laparotomy and duodenocephalopancreasectomy after one month. The definitive histological examination revealed an insulinoma (T3N1MO, AGCC VII G1) with a low replicative index (Ki67: 2%). Conclusions: This report describes a case of malignant insulinoma responsive to octreotide analogs administered pre- operatively in order to try to prevent hypoglycemia. The response to octreotide analogs is not predictable and should be initially assessed under strict clinical surveillance. -

Adrenal Metastasis from an Esophageal Squamous Cell Carcinoma - a Case Report and Review of Literature
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 15, Issue 10 Ver. VII (October. 2016), PP 20-22 www.iosrjournals.org Adrenal Metastasis from an Esophageal Squamous Cell Carcinoma - A Case Report and Review of Literature Prof. Subbiah Shanmugam MS Mch1, Dr Sujay Susikar MS Mch2, Dr. H Prasanna Srinivasa Rao Mch Post Graduate2 1,2Department Of Surgical Oncology, Centre For Oncology, Government Royapettah Hospital & Kilpauk Medical College, Chennai, India Abstract: Adrenal metastasis from esophageal carcinoma is quite uncommon. The identification of adrenal metastasis and their differentiation from incidentally detected benign adrenal tumors is challenging especially when functional imaging facilities are unavailable. Here we present a case report of a 43 year old male presenting with adrenal metastasis from an esophageal squamous cell carcinoma. The use of minimally invasive surgery to confirm the metastatic nature of disease in a resource limited setup has been described. Keywords: adrenal metastasis, adrenalectomy, esophageal squamous cell carcinoma I. Introduction Adrenal metastases have been reported in various malignancies; most commonly from cancers of lung, breast but uncommonly from esophageal primary. The diagnostic difficulties in the identification of adrenal secondaries are due to the small size of the lesion, difficulty in differentiating benign from malignant adrenal lesions based on computed tomography findings alone and the anatomical position of adrenal making it difficult to target for biopsy under image guidance. The functional scans (PET CT) not only reliably differentiate metastatic adrenal lesions, but also light up other areas of metastasis. Such information is definitely needed before deciding on the intent of treatment and the surgery for the primary lesion. -

A Study on the Variations of Arterial Supply to Adrenal Gland
K Naga Vidya Lakshmi and Mahesh Dhoot / International Journal of Biomedical and Advance Research 2016; 7(8): 373-375. 373 International Journal of Biomedical and Advance Research ISSN: 2229-3809 (Online); 2455-0558 (Print) Journal DOI: 10.7439/ijbar CODEN: IJBABN Original Research Article A study on the variations of arterial supply to adrenal gland K Naga Vidya Lakshmi* and Mahesh Dhoot Department of Anatomy, RKDF Medical College Hospital & Research Centre, Bhopal, India *Correspondence Info: Dr. K Naga Vidya Lakshmi, Room No: 208, Staff Quarters, RKDF Medical College Campus, Jatkhedi, Bhopal, India E-mail: [email protected] Abstract Introduction: Adrenal glands are richly vascular and get their arterial supply by means of three arteries namely superior, middle, inferior suprarenal arteries. The inferior phrenic artery gives off the superior branch, while middle branch arises directly from the abdominal aorta, and the inferior suprarenal branch is given off by the renal artery. Materials And Methods: The study was conducted in 15 formalin fixed cadavers obtained from the department of Anatomy and are carefully dissected to observe the arterial supply of both right and left adrenal glands. Observations: It has been noted that variations were observed in four cases out of 30 adrenal glands, two cases showed variations on right side and in two cases variation is seen on left side. First case on the right side, both MSA and ISA are given off by ARA. In the second case on right side IPA is given off by RA and from the junction of these two arteries MSA was given. In third case on left side MSA is given by the coeliac trunk and ISA is from ARA, in fourth case on left side ISA is originated from ARA. -

Pyrexia of Unknown Origin. Presenting Sign of Hypothalamic Hypopituitarism R
Postgrad Med J: first published as 10.1136/pgmj.57.667.310 on 1 May 1981. Downloaded from Postgraduate Medical Journal (May 1981) 57, 310-313 Pyrexia of unknown origin. Presenting sign of hypothalamic hypopituitarism R. MARILUS* A. BARKAN* M.D. M.D. S. LEIBAt R. ARIE* M.D. M.D. I. BLUM* M.D. *Department of Internal Medicine 'B' and tDepartment ofEndocrinology, Beilinson Medical Center, Petah Tiqva, The Sackler School of Medicine, Tel Aviv University, Ramat Aviv, Israel Summary least 10 such admissions because offever of unknown A 62-year-old man was admitted to hospital 10 times origin had been recorded. During this period, he over 12 years because of pyrexia of unknown origin. was extensively investigated for possible infectious, Hypothalamic hypopituitarism was diagnosed by neoplastic, inflammatory and collagen diseases, but dynamic tests including clomiphene, LRH, TRH and the various tests failed to reveal the cause of theby copyright. chlorpromazine stimulation. Lack of ACTH was fever. demonstrated by long and short tetracosactrin tests. A detailed past history of the patient was non- The aetiology of the disorder was believed to be contributory. However, further questioning at a previous encephalitis. later period of his admission revealed interesting Following substitution therapy with adrenal and pertinent facts. Twelve years before the present gonadal steroids there were no further episodes of admission his body hair and sex activity had been fever. normal. At that time he had an acute febrile illness with severe headache which lasted for about one Introduction week. He was not admitted to hospital and did not http://pmj.bmj.com/ Pyrexia of unknown origin (PUO) may present receive any specific therapy. -

Chronic Fatigue: Is It Endocrinology?
I ORIGINAL PAPERS Chronic fatigue: is it endocrinology? Kate M Evans, Daniel E Flanagan and Terence J Wilkin Kate M Evans ABSTRACT – Fatigue and stress-related illnesses imal physical signs where initial investigations are MRCP, Consultant often become diagnoses of exclusion after exten- normal. It is possible to reassure the individual that Physician sive investigation. ‘Tired all the time’ is a frequent there is no significant disease at that point but there Daniel E reason for referral to the endocrine clinic, the are no clear data to address the question of whether Flanagan implicit question being – is there a subtle overt pathology will develop in the future. This paper FRCP, Consultant endocrine pathology contributing to the patient’s reports audit findings for a cohort of patients seen Physician symptoms? Often initial assessment suggests not in an endocrinology clinic with fatigue, including but there are no clear data to address the ques- Terence J Wilkin outcome data at five or more years later. FRCP, Professor of tion of whether overt pathology will develop in the Medicine future. This study observed outcomes after five Subjects and methods years in 101 consecutive and unselected referrals Department of to secondary care for ‘fatigue?cause’, where initial Consecutive and unselected referrals from primary Endocrinology and assessment did not suggest treatable endocrine care to the endocrinology service over a four-year Metabolism, Peninsula Medical pathology. The findings suggest that the clinical period (1995–99) were identified from the clinic School, Plymouth diagnosis of fatigue, based on history and tests to database. Referrals were included in the dataset if the and Derriford exclude anaemia, hypothyroidism and diabetes, is primary reason for referral or primary symptom was Hospital, Plymouth secure: these patients do not subsequently fatigue. -
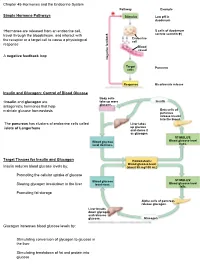
Chapter 45-Hormones and the Endocrine System Pathway Example – Simple Hormone Pathways Stimulus Low Ph in Duodenum
Chapter 45-Hormones and the Endocrine System Pathway Example – Simple Hormone Pathways Stimulus Low pH in duodenum •Hormones are released from an endocrine cell, S cells of duodenum travel through the bloodstream, and interact with secrete secretin ( ) Endocrine the receptor or a target cell to cause a physiological cell response Blood vessel A negative feedback loop Target Pancreas cells Response Bicarbonate release Insulin and Glucagon: Control of Blood Glucose Body cells •Insulin and glucagon are take up more Insulin antagonistic hormones that help glucose. maintain glucose homeostasis Beta cells of pancreas release insulin into the blood. The pancreas has clusters of endocrine cells called Liver takes islets of Langerhans up glucose and stores it as glycogen. STIMULUS: Blood glucose Blood glucose level level declines. rises. Target Tissues for Insulin and Glucagon Homeostasis: Blood glucose level Insulin reduces blood glucose levels by: (about 90 mg/100 mL) Promoting the cellular uptake of glucose Blood glucose STIMULUS: Slowing glycogen breakdown in the liver level rises. Blood glucose level falls. Promoting fat storage Alpha cells of pancreas release glucagon. Liver breaks down glycogen and releases glucose. Glucagon Glucagon increases blood glucose levels by: Stimulating conversion of glycogen to glucose in the liver Stimulating breakdown of fat and protein into glucose Diabetes Mellitus Type I diabetes mellitus (insulin-dependent) is an autoimmune disorder in which the immune system destroys pancreatic beta cells Type II diabetes -

Endocrinology Test List Endocrinology Test List
For Endocrinologists Endocrinology Test List Endocrinology Test List Extensive Capabilities Managing patients with endocrine disorders is complex. Having access to the right test for the right patient is key. With a legacy of expertise in endocrine laboratory diagnostics, Quest Diagnostics offers an extensive menu of laboratory tests across the spectrum of endocrine disorders. This test list highlights the extensive menu of laboratory diagnostic tests we offer, including highly specialized tests and those performed using highly specific and sensitive mass spectrometry detection. It is conveniently organized by glandular function or common endocrine disorder, making it easy for you to identify the tests you need to care for the patients you treat. Comprehensive Care Quest Diagnostics Nichols Institute has been pioneering state-of-the-art endocrine testing for over four decades. Our commitment to innovative diagnostics and our dedication to quality and service means we deliver solutions that enable you to make informed clinical decisions for comprehensive patient management. We strive to remain at the forefront of innovation in endocrine testing so you can deliver the highest level of patient care. Abbreviations and Footnotes NDM, neonatal diabetes mellitus; MODY, maturity-onset diabetes of the young; CH, congenital hyperinsulinism; MSUD, maple syrup urine disease; IHH, idiopathic hypogonadotropic hypogonadism; BBS, Bardet-Biedl syndrome; OI, osteogenesis imperfecta; PKD, polycystic kidney disease; OPPG, osteoporosis-pseudoglioma syndrome; CPHD, combined pituitary hormone deficiency; GHD, growth hormone deficiency. The tests highlighted in green are performed using highly specific and sensitive mass spectrometry detection. Panels that include a test(s) performed using mass spectrometry are highlighted in yellow. For tests highlighted in blue, refer to the Athena Diagnostics website (athenadiagnostics.com/content/test-catalog) for test information. -

Hypothalamic-Pituitary Axes
Hypothalamic-Pituitary Axes Hypothalamic Factors Releasing/Inhibiting Pituitary Anterior Pituitary Hormones Circulating ACTH PRL GH Hormones February 11, 2008 LH FSH TSH Posterior Target Pituitary Gland and Hormones Tissue Effects ADH, oxytocin The GH/IGF-I Axis Growth Hormone Somatostatin GHRH Hypothalamus • Synthesized in the anterior lobe of the pituitary gland in somatotroph cells PITUITARY • ~75% of GH in the pituitary and in circulation is Ghrelin 191 amino acid single chain peptide, 2 intra-molecular disulfide bonds GH Weight; 22kD • Amount of GH secreted: IGF-I Women: 500 µg/m2/day Synthesis IGF- I Men: 350 µg/m2/day LIVER Local IGF-I Synthesis CIRCULATION GH Secretion: Primarily Pulsatile Pattern of GH Secretion Regulation by two hypothalamic in a Healthy Adult hormones 25 Sleep 20 Growth - SMS Hormone 15 Somatostatin Releasing GHRH + GH (µg/L) Hormone Inhibitory of 10 Stimulatory of GH Secretion GH Secretion 05 0 GHRH induces GH Somatostatin: Decreases to allow 0900 2100 0900 synthesis and secretion Clocktime GH secretory in somatotrophs Bursts GH From: “Acromegaly” by Alan G. Harris, M.D. 1 Other Physiological Regulators of GH Secretion Pharmacologic Agents Used to Stimulate GH Secretion Amino Sleep Exercise Stress Acids Fasting Glucose Stimulate hypothalamic GHRH or Inhibit Somatostatin Hypothalamus GHRH SMS Hypoglycemia(Insulin) Pituitary L-dopa Arginine Clonidine GHRH + - SMS Pyridostigmine GH Target Tissues Metabolic & Growth Promoting GH Effects IGF-I Insulin-like growth factor I (IGF-I) Major Determinants of Circulating -

Current Medicine: Current Aspects of Thyroid Disease
CURRENT MEDICINE CM CURRENT ASPECTS OF THYROID DISEASE J.H. Lazarus, Department of Medicine, University of Wales College of Medicine, Cardiff INTRODUCTION solved) the details of the immune dysfunction in these During the past two decades or so, advances in immunology conditions. Since the description of the HLA system and and molecular biology have contributed to a substantial the association of certain haplotypes with autoimmune increase in our understanding of many aspects of the endocrine disease, including Graves’ disease and Hashimoto’s pathophysiology of thyroid disease.1 This review will thyroiditis, it was anticipated that the immunogenetics of indicate some of these advances prior to illustrating their these diseases would shed definitive light on their significance in clinical practice. Emphasis is placed on pathogenesis. This hope has only been partially achieved, recent developments and it is not intended to provide full and the relative risk of the HLA haplotype has been low; clinical descriptions. other factors must be sought. Developments in the understanding of the interaction between the T lymphocyte THYROID HORMONE ACTION and the presentation of antigen (e.g. co-stimulatory signals) Since the discovery that the thyroid hormone receptor is have enabled further immunological insight.5 However, derived from the erb c oncogene, it has been realised that the role of environmental factors should not be overlooked. there are two receptor types, alpha and beta, whose genes The ambient iodine concentration may affect the incidence are located on chromosomes 3 and 17 respectively.2 There of autoimmune thyroid disease, and iodine has documented is now known to be differential tissue distribution of the immune effects in the thyroid gland both in vitro and in active TRs alpha 1 and beta 1 and 2.