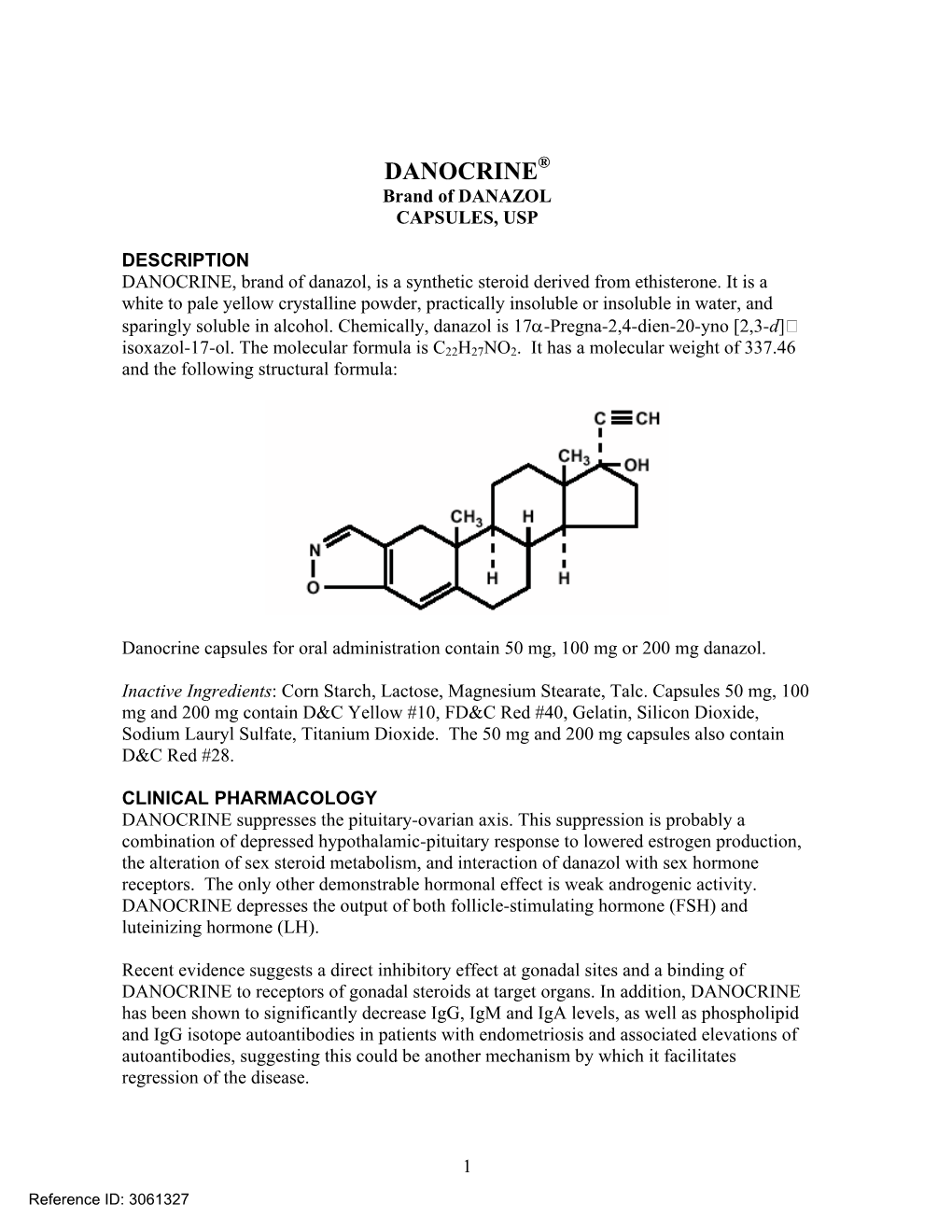
DANOCRINE Brand of DANAZOL CAPSULES, USP
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Detectx® Serum 17Β-Estradiol Enzyme Immunoassay Kit
DetectX® Serum 17β-Estradiol Enzyme Immunoassay Kit 1 Plate Kit Catalog Number KB30-H1 5 Plate Kit Catalog Number KB30-H5 Species Independent Sample Types Validated: Multi-Species Serum and Plasma Please read this insert completely prior to using the product. For research use only. Not for use in diagnostic procedures. www.ArborAssays.com KB30-H WEB 210308 TABLE OF CONTENTS Background 3 Assay Principle 4 Related Products 4 Supplied Components 5 Storage Instructions 5 Other Materials Required 6 Precautions 6 Sample Types 7 Sample Preparation 7 Reagent Preparation 8 Assay Protocol 9 Calculation of Results 10 Typical Data 10-11 Validation Data Sensitivity, Linearity, etc. 11-13 Samples Values and Cross Reactivity 14 Warranty & Contact Information 15 Plate Layout Sheet 16 ® 2 EXPECT ASSAY ARTISTRY KB30-H WEB 210308 BACKGROUND 17β-Estradiol, C18H24O2, also known as E2 or oestradiol (1, 3, 5(10)-Estratrien-3, 17β-diol) is a key regulator of growth, differentiation, and function in a wide array of tissues, including the male and female reproductive tracts, mammary gland, brain, skeletal and cardiovascular systems. The predominant biological effects of E2 are mediated through two distinct intracellular receptors, ERα and ERβ, each encoded by unique genes possessing the functional domain characteristics of the steroid/thyroid hormone superfamily of nuclear receptors1. ERα is the predominant form expressed in the breast, uterus, cervix, and vagina. ERβ exhibits a more limited pattern and is primarily expressed in the ovary, prostate, testis, spleen, lung, hypothalamus, and thymus2. Estradiol also influences bone growth, brain development and maturation, and food intake3, and it is also critical in maintaining organ functions during severe trauma4,5. -

A Guide to Feminizing Hormones – Estrogen
1 | Feminizing Hormones A Guide to Feminizing Hormones Hormone therapy is an option that can help transgender and gender-diverse people feel more comfortable in their bodies. Like other medical treatments, there are benefits and risks. Knowing what to expect will help us work together to maximize the benefits and minimize the risks. What are hormones? Hormones are chemical messengers that tell the body’s cells how to function, when to grow, when to divide, and when to die. They regulate many functions, including growth, sex drive, hunger, thirst, digestion, metabolism, fat burning & storage, blood sugar, cholesterol levels, and reproduction. What are sex hormones? Sex hormones regulate the development of sex characteristics, including the sex organs such as genitals and ovaries/testicles. Sex hormones also affect the secondary sex characteristics that typically develop at puberty, like facial and body hair, bone growth, breast growth, and voice changes. There are three categories of sex hormones in the body: • Androgens: testosterone, dehydroepiandrosterone (DHEA), dihydrotestosterone (DHT) • Estrogens: estradiol, estriol, estrone • Progestin: progesterone Generally, “males” tend to have higher androgen levels, and “females” tend to have higher levels of estrogens and progestogens. What is hormone therapy? Hormone therapy is taking medicine to change the levels of sex hormones in your body. Changing these levels will affect your hair growth, voice pitch, fat distribution, muscle mass, and other features associated with sex and gender. Feminizing hormone therapy can help make the body look and feel less “masculine” and more “feminine" — making your body more closely match your identity. What medicines are involved? There are different kinds of medicines used to change the levels of sex hormones in your body. -

Hormonal Treatment Strategies Tailored to Non-Binary Transgender Individuals
Journal of Clinical Medicine Review Hormonal Treatment Strategies Tailored to Non-Binary Transgender Individuals Carlotta Cocchetti 1, Jiska Ristori 1, Alessia Romani 1, Mario Maggi 2 and Alessandra Daphne Fisher 1,* 1 Andrology, Women’s Endocrinology and Gender Incongruence Unit, Florence University Hospital, 50139 Florence, Italy; [email protected] (C.C); jiska.ristori@unifi.it (J.R.); [email protected] (A.R.) 2 Department of Experimental, Clinical and Biomedical Sciences, Careggi University Hospital, 50139 Florence, Italy; [email protected]fi.it * Correspondence: fi[email protected] Received: 16 April 2020; Accepted: 18 May 2020; Published: 26 May 2020 Abstract: Introduction: To date no standardized hormonal treatment protocols for non-binary transgender individuals have been described in the literature and there is a lack of data regarding their efficacy and safety. Objectives: To suggest possible treatment strategies for non-binary transgender individuals with non-standardized requests and to emphasize the importance of a personalized clinical approach. Methods: A narrative review of pertinent literature on gender-affirming hormonal treatment in transgender persons was performed using PubMed. Results: New hormonal treatment regimens outside those reported in current guidelines should be considered for non-binary transgender individuals, in order to improve psychological well-being and quality of life. In the present review we suggested the use of hormonal and non-hormonal compounds, which—based on their mechanism of action—could be used in these cases depending on clients’ requests. Conclusion: Requests for an individualized hormonal treatment in non-binary transgender individuals represent a future challenge for professionals managing transgender health care. For each case, clinicians should balance the benefits and risks of a personalized non-standardized treatment, actively involving the person in decisions regarding hormonal treatment. -

Gender-Affirming Hormone Therapy
GENDER-AFFIRMING HORMONE THERAPY Julie Thompson, PA-C Medical Director of Trans Health, Fenway Health March 2020 fenwayhealth.org GOALS AND OBJECTIVES 1. Review process of initiating hormone therapy through the informed consent model 2. Provide an overview of masculinizing and feminizing hormone therapy 3. Review realistic expectations and benefits of hormone therapy vs their associated risks 4. Discuss recommendations for monitoring fenwayhealth.org PROTOCOLS AND STANDARDS OF CARE fenwayhealth.org WPATH STANDARDS OF CARE, 2011 The criteria for hormone therapy are as follows: 1. Well-documented, persistent (at least 6mo) gender dysphoria 2. Capacity to make a fully informed decision and to consent for treatment 3. Age of majority in a given country 4. If significant medical or mental health concerns are present, they must be reasonably well controlled fenwayhealth.org INFORMED CONSENT MODEL ▪ Requires healthcare provider to ▪ Effectively communicate benefits, risks and alternatives of treatment to patient ▪ Assess that the patient is able to understand and consent to the treatment ▪ Informed consent model does not preclude mental health care! ▪ Recognizes that prescribing decision ultimately rests with clinical judgment of provider working together with the patient ▪ Recognizes patient autonomy and empowers self-agency ▪ Decreases barriers to medically necessary care fenwayhealth.org INITIAL VISITS ▪ Review history of gender experience and patient’s goals ▪ Document prior hormone use ▪ Assess appropriateness for gender affirming medical -

Anabolic Steroids/Androgens Pa Summary
ANABOLIC STEROIDS/ANDROGENS PA SUMMARY PREFERRED Anadrol-50, Danazol, Fluoxymesterone, Methitest, Oxandrolone, Testosterone Cypionate Injection, Testosterone Enanthate Injection NON-PREFERRED Android, Testred LENGTH OF AUTHORIZATION: Varies NOTE: All preferred and non-preferred agents require prior authorization. See PA criteria labeled “Topical Testosterone” for Androderm, Androgel, Striant, and Testim. The criteria details below are for the outpatient pharmacy program. If an injectable medication is being administered in a physician’s office then the criteria information below does not apply. Instead, the physician’s office must bill this drug through the DCH physician’s injectable program and not the outpatient pharmacy program. Information regarding the physician’s injectable program can be located at www.mmis.georgia.gov. PA CRITERIA: For Anadrol-50 Approvable for the following diagnoses: anemia caused by deficient red blood cell production, acquired or congenital aplastic anemia, myelofibrosis, hypoplastic anemia due to administration of myelotoxic drugs Also approvable for HIV or AIDS wasting when significant weight loss is documented in members currently receiving nutritional support For Danazol Approvable for the following diagnoses: endometriosis, fibrocystic breast disease, hereditary angioedema For Fluoxymesterone, Methyltestosterone (Android, Methitest, Testred), Testosterone Cypionate or Enanthate Injection Approvable in male members 12 years of age or older for the following diagnoses: primary hypogonadism, secondary -

St John's Institute of Dermatology
St John’s Institute of Dermatology Topical steroids This leaflet explains more about topical steroids and how they are used to treat a variety of skin conditions. If you have any questions or concerns, please speak to a doctor or nurse caring for you. What are topical corticosteroids and how do they work? Topical corticosteroids are steroids that are applied onto the skin and are used to treat a variety of skin conditions. The type of steroid found in these medicines is similar to those produced naturally in the body and they work by reducing inflammation within the skin, making it less red and itchy. What are the different strengths of topical corticosteroids? Topical steroids come in a number of different strengths. It is therefore very important that you follow the advice of your doctor or specialist nurse and apply the correct strength of steroid to a given area of the body. The strengths of the most commonly prescribed topical steroids in the UK are listed in the table below. Table 1 - strengths of commonly prescribed topical steroids Strength Chemical name Common trade names Mild Hydrocortisone 0.5%, 1.0%, 2.5% Hydrocortisone Dioderm®, Efcortelan®, Mildison® Moderate Betamethasone valerate 0.025% Betnovate-RD® Clobetasone butyrate 0.05% Eumovate®, Clobavate® Fluocinolone acetonide 0.001% Synalar 1 in 4 dilution® Fluocortolone 0.25% Ultralanum Plain® Fludroxycortide 0.0125% Haelan® Tape Strong Betamethasone valerate 0.1% Betnovate® Diflucortolone valerate 0.1% Nerisone® Fluocinolone acetonide 0.025% Synalar® Fluticasone propionate 0.05% Cutivate® Hydrocortisone butyrate 0.1% Locoid® Mometasone furoate 0.1% Elocon® Very strong Clobetasol propionate 0.1% Dermovate®, Clarelux® Diflucortolone valerate 0.3% Nerisone Forte® 1 of 5 In adults, stronger steroids are generally used on the body and mild or moderate steroids are used on the face and skin folds (armpits, breast folds, groin and genitals). -

Quantitative High-Throughput Profiling of Environmental Chemicals and Drugs That Modulate Farnesoid X Receptor
OPEN Quantitative High-Throughput Profiling of SUBJECT AREAS: Environmental Chemicals and Drugs that SCREENING SMALL MOLECULES Modulate Farnesoid X Receptor Chia-Wen Hsu1, Jinghua Zhao1, Ruili Huang1, Jui-Hua Hsieh2, Jon Hamm3, Xiaoqing Chang3, Keith Houck4 Received & Menghang Xia1 27 June 2014 Accepted 1National Center for Advancing Translational Sciences, National Institutes of Health, Bethesda, MD, 2Division of the National 29 August 2014 Toxicology Program, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC, 3Integrated Laboratory Systems, Inc., Morrisville, NC, 4U.S. Environmental Protection Agency, Research Triangle Park, NC. Published 26 September 2014 The farnesoid X receptor (FXR) regulates the homeostasis of bile acids, lipids, and glucose. Because endogenous chemicals bind and activate FXR, it is important to examine which xenobiotic compounds Correspondence and would disrupt normal receptor function. We used a cell-based human FXR b-lactamase (Bla) reporter gene assay to profile the Tox21 10K compound collection of environmental chemicals and drugs. requests for materials Structure-activity relationships of FXR-active compounds revealed by this screening were then compared should be addressed to against the androgen receptor, estrogen receptor a, peroxisome proliferator-activated receptors d and c, and M.X. ([email protected]. the vitamin D receptor. We identified several FXR-active structural classes including anthracyclines, gov) benzimidazoles, dihydropyridines, pyrethroids, retinoic acids, and vinca alkaloids. Microtubule inhibitors potently decreased FXR reporter gene activity. Pyrethroids specifically antagonized FXR transactivation. Anthracyclines affected reporter activity in all tested assays, suggesting non-specific activity. These results provide important information to prioritize chemicals for further investigation, and suggest possible modes of action of compounds in FXR signaling. -

Z:\My Documents\WPDOCS\IACUC
ZOONOTIC DISEASES OF LABORATORY, AGRICULTURAL, AND WILDLIFE ANIMALS July, 2007 Michael S. Rand, DVM, DACLAM University Animal Care University of Arizona PO Box 245092 Tucson, AZ 85724-5092 (520) 626-6705 E-mail: [email protected] http://www.ahsc.arizona.edu/uac Table of Contents Introduction ............................................................................................................................................. 3 Amebiasis ............................................................................................................................................... 5 B Virus .................................................................................................................................................... 6 Balantidiasis ........................................................................................................................................ 6 Brucellosis ........................................................................................................................................ 6 Campylobacteriosis ................................................................................................................................ 7 Capnocytophagosis ............................................................................................................................ 8 Cat Scratch Disease ............................................................................................................................... 9 Chlamydiosis ..................................................................................................................................... -

Small Molecules in Solution Has Rarely Been Reported, However, As a General Guide We Recommend Storage in DMSO at -20°C
(Z)-Guggulsterone Small Molecules Retinoic acid receptor (RAR) pathway inhibitor; Inhibits farnesoid X receptor (FXR) Catalog # 73702 1 mg Product Description (Z)-Guggulsterone is a plant steroid found in the resin of the guggul plant Commiphora mukul that acts as a selective antagonist of farnesoid X receptor (FXR; Cui et al.). It decreases chenodeoxycholic acid (CDCA)-induced FXR activation (IC ₅₀ = 10 µM) in the presence of 100 µM CDCA (Urizar et al.; Cui et al.). Molecular Name: (Z)-Guggulsterone Alternative Names: Not applicable CAS Number: 39025-23-5 Chemical Formula: C₂₁H₂₈O₂ Molecular Weight: 312.5 g/mol Purity: ≥ 95% Chemical Name: (8R,9S,10R,13S,14S,17Z)-17-ethylidene-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15- decahydrocyclopenta[a]phenanthrene-3,16-dione Structure: Properties Physical Appearance: A crystalline solid Storage: Product stable at -20°C as supplied. Protect product from prolonged exposure to light. For long-term storage store with a desiccant. For product expiry date, please contact [email protected]. Solubility: · DMSO ≤ 800 µM · Absolute ethanol ≤ 3.2 µM · DMF ≤ 30 mM For example, to prepare a 10 mM stock solution in DMF, resuspend 1 mg in 320 μL of DMF. Prepare stock solution fresh before use. Information regarding stability of small molecules in solution has rarely been reported, however, as a general guide we recommend storage in DMSO at -20°C. Aliquot into working volumes to avoid repeated freeze-thaw cycles. The effect of storage of stock solution on compound performance should be tested for each application. Compound has low solubility in aqueous media. -

Steroids and Other Appearance and Performance Enhancing Drugs (Apeds) Research Report
Research Report Revised Febrero 2018 Steroids and Other Appearance and Performance Enhancing Drugs (APEDs) Research Report Table of Contents Steroids and Other Appearance and Performance Enhancing Drugs (APEDs) Research Report Introduction What are the different types of APEDs? What is the history of anabolic steroid use? Who uses anabolic steroids? Why are anabolic steroids misused? How are anabolic steroids used? What are the side effects of anabolic steroid misuse? How does anabolic steroid misuse affect behavior? What are the risks of anabolic steroid use in teens? How do anabolic steroids work in the brain? Are anabolic steroids addictive? How are anabolic steroids tested in athletes? What can be done to prevent steroid misuse? What treatments are effective for anabolic steroid misuse? Where can I get further information about steroids? References Page 1 Steroids and Other Appearance and Performance Enhancing Drugs (APEDs) Research Report Esta publicación está disponible para su uso y puede ser reproducida, en su totalidad, sin pedir autorización al NIDA. Se agradece la citación de la fuente, de la siguiente manera: Fuente: Instituto Nacional sobre el Abuso de Drogas; Institutos Nacionales de la Salud; Departamento de Salud y Servicios Humanos de los Estados Unidos. Introduction Appearance and performance enhancing drugs (APEDs) are most often used by males to improve appearance by building muscle mass or to enhance athletic performance. Although they may directly and indirectly have effects on a user’s mood, they do not produce a euphoric high, which makes APEDs distinct from other drugs such as cocaine, heroin, and marijuana. However, users may develop a substance use disorder, defined as continued use despite adverse consequences. -

Ep 2446888 A2
(19) & (11) EP 2 446 888 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 02.05.2012 Bulletin 2012/18 A61K 31/569 (2006.01) (21) Application number: 11010272.0 (22) Date of filing: 12.07.2006 (84) Designated Contracting States: (71) Applicant: DMI Biosciences, Inc. AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Englewood, CO 80110-3948 (US) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI SK TR (72) Inventor: Bar-Or, David Designated Extension States: Englewood, Colorado 80110 (US) AL BA HR MK RS (74) Representative: Schaeberle, Steffen (30) Priority: 12.07.2005 US 69872305 P Hoefer & Partner 24.08.2005 US 71115705 P Patentanwälte 24.08.2005 US 71115805 P Pilgersheimer Strasse 20 81543 München (DE) (62) Document number(s) of the earlier application(s) in accordance with Art. 76 EPC: Remarks: 06787393.5 / 1 919 290 This application was filed on 29-12-2011 as a divisional application to the application mentioned under INID code 62. (54) Use of danazol for the treatment of uveitis (57) The present invention relates to the treatment of an inflammatory disease or condition of the eye such as uveitis with danazof or a pharmacologically-acceptable salt or ester thereof. EP 2 446 888 A2 Printed by Jouve, 75001 PARIS (FR) EP 2 446 888 A2 Description FIELD OF THE INVENTION 5 [0001] The present invention relates to the treatment of diseases and conditions with an effective amount of a steroid having those formulas given below, or a pharmacologically-acceptable salt or ester thereof. -

Hirsutism and Polycystic Ovary Syndrome (PCOS)
Hirsutism and Polycystic Ovary Syndrome (PCOS) A Guide for Patients PATIENT INFORMATION SERIES Published by the American Society for Reproductive Medicine under the direction of the Patient Education Committee and the Publications Committee. No portion herein may be reproduced in any form without written permission. This booklet is in no way intended to replace, dictate or fully define evaluation and treatment by a qualified physician. It is intended solely as an aid for patients seeking general information on issues in reproductive medicine. Copyright © 2016 by the American Society for Reproductive Medicine AMERICAN SOCIETY FOR REPRODUCTIVE MEDICINE Hirsutism and Polycystic Ovary Syndrome (PCOS) A Guide for Patients Revised 2016 A glossary of italicized words is located at the end of this booklet. INTRODUCTION Hirsutism is the excessive growth of facial or body hair on women. Hirsutism can be seen as coarse, dark hair that may appear on the face, chest, abdomen, back, upper arms, or upper legs. Hirsutism is a symptom of medical disorders associated with the hormones called androgens. Polycystic ovary syndrome (PCOS), in which the ovaries produce excessive amounts of androgens, is the most common cause of hirsutism and may affect up to 10% of women. Hirsutism is very common and often improves with medical management. Prompt medical attention is important because delaying treatment makes the treatment more difficult and may have long-term health consequences. OVERVIEW OF NORMAL HAIR GROWTH Understanding the process of normal hair growth will help you understand hirsutism. Each hair grows from a follicle deep in your skin. As long as these follicles are not completely destroyed, hair will continue to grow even if the shaft, which is the part of the hair that appears above the skin, is plucked or removed.