Spatiotemporal Variation in Wetland Fish Assemblages in the Western
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Eco-Hydrology and Chemical Pollution of Western Ghats
Eco-hydrology and Chemical Pollution of Western Ghats Dr.Mathew Koshy M..Sc., M.Phil., Ph.D. Reader and Research Guide in Chemistry, Bishop Moore College, Mavelikara.Kerala Eco-hydrology Eco-hydrology is a new integrative science that involves finding solutions to issues surrounding water, people, and the environment. One of the fundamental concepts involved in eco-hydrology is that the timing and availability of freshwater is intimately linked to ecosystem processes, and the goods and services provided by fresh waters to societies. This means that emphasis is placed on the hydrological cycle and its effects on ecological processes and human well-being. Limnology Limnology is the science that deals with the physical, chemical and biological properties and features of fresh waters. A professional who studies fresh water systems is a limnologist. Lotic System: The lotic environment is consisting of all inland waters in which entire water body continually flows in a definite direction. etc. rivers streams. Lentic system: The lentic environment has been including all inland waters in which water has been not continually flowing in a definite direction. Standing waters Western Ghats The Western Ghats hill range extends along the west coast of India, covering an area of 160,000 square kilometers. The presence of these hills creates major precipitation gradients that strongly influence regional climate, hydrology and the distribution of vegetation types and endemic plants. Biodiversity Although the total area is less than 6 percent of the land area of India, the Western Ghats contains more than 30 percent of all plant, fish, fauna, bird, and mammal species found in India. -

District Survey Report of Madurai District
Content 1.0 Preamble ................................................................................................................. 1 2.0 Introduction .............................................................................................................. 1 2.1 Location ............................................................................................................ 2 3.0 Overview of Mining Activity In The District .............................................................. 3 4.0 List of Mining Leases details ................................................................................... 5 5.0 Details of the Royalty or Revenue received in last Three Years ............................ 36 6.0 Details of Production of Sand or Bajri Or Minor Minerals In Last Three Years ..... 36 7.0 Process of deposition of Sediments In The River of The District ........................... 36 8.0 General Profile of Maduari District ....................................................................... 27 8.1 History ............................................................................................................. 28 8.2 Geography ....................................................................................................... 28 8.3 Taluk ................................................................................................................ 28 8.2 Blocks .............................................................................................................. 29 9.0 Land Utilization Pattern In The -
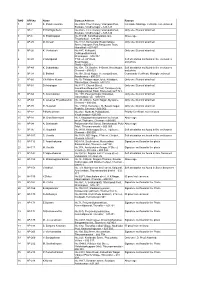
SNO APP.No Name Contact Address Reason 1 AP-1 K
SNO APP.No Name Contact Address Reason 1 AP-1 K. Pandeeswaran No.2/545, Then Colony, Vilampatti Post, Intercaste Marriage certificate not enclosed Sivakasi, Virudhunagar – 626 124 2 AP-2 P. Karthigai Selvi No.2/545, Then Colony, Vilampatti Post, Only one ID proof attached. Sivakasi, Virudhunagar – 626 124 3 AP-8 N. Esakkiappan No.37/45E, Nandhagopalapuram, Above age Thoothukudi – 628 002. 4 AP-25 M. Dinesh No.4/133, Kothamalai Road,Vadaku Only one ID proof attached. Street,Vadugam Post,Rasipuram Taluk, Namakkal – 637 407. 5 AP-26 K. Venkatesh No.4/47, Kettupatti, Only one ID proof attached. Dokkupodhanahalli, Dharmapuri – 636 807. 6 AP-28 P. Manipandi 1stStreet, 24thWard, Self attestation not found in the enclosures Sivaji Nagar, and photo Theni – 625 531. 7 AP-49 K. Sobanbabu No.10/4, T.K.Garden, 3rdStreet, Korukkupet, Self attestation not found in the enclosures Chennai – 600 021. and photo 8 AP-58 S. Barkavi No.168, Sivaji Nagar, Veerampattinam, Community Certificate Wrongly enclosed Pondicherry – 605 007. 9 AP-60 V.A.Kishor Kumar No.19, Thilagar nagar, Ist st, Kaladipet, Only one ID proof attached. Thiruvottiyur, Chennai -600 019 10 AP-61 D.Anbalagan No.8/171, Church Street, Only one ID proof attached. Komathimuthupuram Post, Panaiyoor(via) Changarankovil Taluk, Tirunelveli, 627 761. 11 AP-64 S. Arun kannan No. 15D, Poonga Nagar, Kaladipet, Only one ID proof attached. Thiruvottiyur, Ch – 600 019 12 AP-69 K. Lavanya Priyadharshini No, 35, A Block, Nochi Nagar, Mylapore, Only one ID proof attached. Chennai – 600 004 13 AP-70 G. -

Annexure-District Survey Report
TIRUNELVELI DISTRICT PROFILE Tirunelveli district is bounded by Virudhunagar district in the north, Thoothukudi district in the east, in the south by Gulf of Mannar and by Kerala State in the west and Kanniyakumari in the southwest. The District lies between 08º08'09’’N to 09º24'30’’N Latitude, 77º08'30’’E to 77º58'30’’E Longitude and has an areal extent of 6810 sq.km. There are 19 Blocks, 425 Villages and 2579 Habitations in the District. District Map of Tirunelveli District Google Map of Tirunelveli District Administrative Details Tirunelveli district is divided into 9 taluks. The taluks are further divided into 19 blocks, which further divided into 586 villages. Basin and sub-basin The district is part of the composite east flowing river basin,“ Between Vaippar and Nambiar ” as per the Irrigation Atlas of India. Tambarabarani, Vaipar and Nambiar are the important Sub-basins. Drainage Thamarabarani, Nambiar, Chittar and Karamaniar are the important rivers draining the district. amarabarani originating from Papanasam flows thorough the district.The Nambiyar river originates in the eastern slopes of the Western ghats near Nellikalmottai about 9.6 km west of Tirukkurugundi village at an altitude of about 1060 m amsl At the foot of the hills, the river is divided into two arms. The main arm is joined by Tamarabarani at the foothills. Chittar originates near Courtallam and flows through Tenkasi and confluences with Tamarabarani. The hilly terrains have resulted in number of falls in the district. There are three major falls in ManimuttarReservoir catchments area and there are few falls in the Tamarabarani river also. -
![The Legend Marthanda Varma [1] C.Parthiban Sarathi [1] II M.A History, Scott Christian College(Autonomous), Nagercoil](https://docslib.b-cdn.net/cover/8445/the-legend-marthanda-varma-1-c-parthiban-sarathi-1-ii-m-a-history-scott-christian-college-autonomous-nagercoil-488445.webp)
The Legend Marthanda Varma [1] C.Parthiban Sarathi [1] II M.A History, Scott Christian College(Autonomous), Nagercoil
ISSN (Online) 2456 -1304 International Journal of Science, Engineering and Management (IJSEM) Vol 2, Issue 12, December 2017 The legend Marthanda Varma [1] C.Parthiban Sarathi [1] II M.A History, Scott Christian College(Autonomous), Nagercoil. Abstract:-- Marthanda Varma the founder of modern Travancore. He was born in 1705. Anizham Tirunal Marthanda Varma rule of Travancore in 1929. Marthanda Varma headquarters in Kalkulam. Marthanda Varma very important policy in Blood and Iron policy. Marthanda Varma reorganised the financial department the palace of Padmanabhapuram was improved and several new buildings. There was improvement of communication following the opening of new Roads and canals. Irrigation works like the ponmana and puthen dams. Marthanda Varma rulling period very important war in Battle of Colachel. The As the Dutch military team captain Eustachius De Lannoy and our soldiers surrendered in Travancore king. Marthanda Varma asked Dutch captain Delannoy to work for the Travancore army Delannoy accepted to take service under the maharaja Delannoy trained with European style of military drill and tactics. Commander in chief of the Travancore military, locally called as valia kapitaan. This king period Padmanabhaswamy temple in Ottakkal mandapam built in Marthanda Varma. The king decided to donate his recalm to Sri Padmanabha and thereafter rule as the deity's vice regent the dedication took place on January 3, 1750 and thereafter he was referred to as Padmanabhadasa Thrippadidanam. The legend king Marthanda Varma 7 July 1758 is dead. Keywords:-- Marthanda Varma, Battle of Colachel, Dutch military captain Delannoy INTRODUCTION English and the Dutch and would have completely quelled the rebels but for the timidity and weakness of his uncle the Anizham Tirunal Marthanda Varma was a ruler of the king who completed him to desist. -

Land and Building Situated in Theni District-Periyakulam Registration District- Andipatti Taluk, Kadamalaigundu Sub Registration
Retail Lending and Payment Group (South Zonal Office/Branch):Axis Bank-RAC, Arcot Plaza, Old No.38, New No.165, Arcot Road, Kodambakkam, Chennai - 600024. Corporate Office:-, “Axis House”, C-2, Wadia International Centre, Pandurang Budhkar Marg, Worli, Mumbai – 400025. Registered Office: “Trishul”, 3rd Floor Opp. Samartheshwar Temple Law Garden, Ellisbridge Ahmedabad – 380006. Public notice for Sale/Auction of immovable properties Under SARFAESI Act read with provision to Rule 8 (6) of the Security Interest (Enforcement) Rules Whereas the Authorized Officer of Axis Bank Ltd. (hereinafter referred to as ‘the Bank’), under Securitisation and Reconstruction of Financial Assets and Enforcement of Security Interest Act, 2002 (in short ‘SARFAESI Act) and in exercise of powers conferred under Section 13(12) read with the Security Interest (Enforcement) Rules, 2002 issued Demand Notice under Sec. 13(2) of SARFAESI Act calling upon the below-mentioned Borrowers/Co-borrowers/mortgagors/Guarantors to repay the amount mentioned in the notice being the amount due together with further interest thereon at the contractual rate plus all costs charges and incidental expenses etc. till the date of payment within 60 days from the date of the said notice. The Borrowers/Co-borrowers/mortgagors/Guarantors having failed to repay the above said amount within the specified period, the authorized officer has taken over physical possession in exercise of powers conferred under Section 13(4) of SARFAESI Act read with Security Interest (Enforcement) Rules, 2002, -

Irrigation Projects of Tamil Nadu from 2001-2021
IRRIGATION PROJECTS OF TAMIL NADU FROM 2001-2021 NAME – VRINDA GUPTA INSTITUTION – K.R. MANGALAM UNIVERSITY 1 ABSTRACT From the ancient times water is always most important for agriculture purpose for growing crops. Since thousand years, humans have relied on agriculture to feed their communities and they have needed irrigation to water their crops. Irrigation includes artificially applying water to the land to enhance the growing of crops. Over the years, irrigation has come in many different forms in countries all over the world. Irrigation projects involves hydraulic structures which collect, convey and deliver water to those areas on which crops are grown. Irrigation projects unit may starts from a small farm unit to those serving extensive areas of millions of hectares. Irrigation projects consist of two types first a small irrigation project and second a large irrigation project. Small irrigation project includes a low diversion or an inexpensive pumping plant along with small channels and some minor control structures. Large irrigation project includes a huge dam, a large storage reservoir, hundreds kilometers of canals, branches and distributaries, control structures and other works. In this paper we discussing about irrigation plan of Tamil Nadu from 2001-2021. INTRODUCTION Water is the important or elixir of life, a precious gift of nature to humans and millions of other species living on the earth. It is hard to find in most part of the world. 4% of India’s land area in Tamil Nadu and inhabited by 6% of India’s population but water resources in India is only 2.5%. In Tamil Nadu, water is a serious limiting factor for agriculture growth which leads to irrigation reduces risk in farming, increases crop productivity, provides higher employment opportunities to the rural areas and increases farmer income. -

The Madras Presidency, with Mysore, Coorg and the Associated States
: TheMADRAS PRESIDENG 'ff^^^^I^t p WithMysore, CooRGAND the Associated States byB. THURSTON -...—.— .^ — finr i Tin- PROVINCIAL GEOGRAPHIES Of IN QJofttell HttinerHitg Blibracg CHARLES WILLIAM WASON COLLECTION CHINA AND THE CHINESE THE GIFT OF CHARLES WILLIAM WASON CLASS OF 1876 1918 Digitized by Microsoft® Cornell University Library DS 485.M27T54 The Madras presidencypresidenc; with MysorMysore, Coor iliiiiliiiiiiilii 3 1924 021 471 002 Digitized by Microsoft® This book was digitized by Microsoft Corporation in cooperation witli Cornell University Libraries, 2007. You may use and print this copy in limited quantity for your personal purposes, but may not distribute or provide access to it (or modified or partial versions of it) for revenue-generating or other commercial purposes. Digitized by Microsoft® Provincial Geographies of India General Editor Sir T. H. HOLLAND, K.C.LE., D.Sc, F.R.S. THE MADRAS PRESIDENCY WITH MYSORE, COORG AND THE ASSOCIATED STATES Digitized by Microsoft® CAMBRIDGE UNIVERSITY PRESS HonBnn: FETTER LANE, E.G. C. F. CLAY, Man^gek (EBiniurBi) : loo, PRINCES STREET Berlin: A. ASHER AND CO. Ji-tipjifl: F. A. BROCKHAUS i^cto Sotfe: G. P. PUTNAM'S SONS iBomlaj sriB Calcutta: MACMILLAN AND CO., Ltd. All rights reserved Digitized by Microsoft® THE MADRAS PRESIDENCY WITH MYSORE, COORG AND THE ASSOCIATED STATES BY EDGAR THURSTON, CLE. SOMETIME SUPERINTENDENT OF THE MADRAS GOVERNMENT MUSEUM Cambridge : at the University Press 1913 Digitized by Microsoft® ffiambttige: PRINTED BY JOHN CLAY, M.A. AT THE UNIVERSITY PRESS. Digitized by Microsoft® EDITOR'S PREFACE "HE casual visitor to India, who limits his observations I of the country to the all-too-short cool season, is so impressed by the contrast between Indian life and that with which he has been previously acquainted that he seldom realises the great local diversity of language and ethnology. -

Cumban-Valley
INDIAN MEDICAL ASSOCIATION TAMILNADU STATE BRANCH FAMILY SECURITY SCHEME Doctor Details Branch : CUMBUM VALLEY Total No. of Doctors : 168 R.No. Dr. Name City Mobile 756 Dr. C RAMASAMY MBBS Theni 9443568134 757 Dr. R MOHANKUMAR MBBS CHINNAMANUR 9842542000 758 Dr. P SATHIANESAN MBBS CHINNAMANUR 9788855359 759 Dr. S CHELLAPANDIAN MBBS Theni 9443501756 1087 Dr. C JEYACHANDRAN MBBS Theni 9443743263 1207 Dr. A VIMALA MBBS Periyakulam 9487352300 1208 Dr. S.R THIRUMALAI MBBS Periyakulam 9786650656 1277 Dr. G RAJ KUMAR MBBS Theni 9442161727 1307 Dr. A SENTHIL KUMAR MBBS Periyakulam 9994658490 1369 Dr. A KALAVATHY MBBS CHINNAMANUR 9600281487 1370 Dr. V SUBBAIAN MBBS Theni 9486227787 1455 Dr. M.J SUDHAKAR MBBS Theni 9943717004 1541 Dr. V JAYALAKSHMI MBBS CHINNAMANUR 9842163486 1545 Dr. N SUNDRA VADIVELAN MBBS CHINNAMANUR 9842163486 2081 Dr. HEMA DAYASEKARAN MBBS Theni 9443918985 2091 Dr. B ALAGAMMAL MBBS Theni 9865347435 2235 Dr. R ARAVAZHI MBBS Theni 9443474418 2476 Dr. R DHOSS MBBS Theni 9443134519 2540 Dr. L.V.K MOORTHY MBBS Periyakulam 9443164851 2543 Dr. H.C. SEKHAR MBBS Theni 9346755759 2649 Dr. S UMA MBBS Theni 9443719462 2650 Dr. B KANNAN MBBS Theni 9443719462 2752 Dr. M ASHOK KUMAR MBBS Theni 9486258680 2919 Dr. A MAHALAKSHMI MBBS Theni 9443593220 2974 Dr. A.V KARUNANIDHI MBBS Theni 9244201599 3028 Dr. S.K KAMARAJ MD.D.C.H., Theni 9487002690 3054 Dr. V PANDIAN MBBS Periyakulam 9894631567 3148 Dr. K.S KUMAR MBBS Periyakulam 9443804300 3290 Dr. S.R SUBRAMANIAN MBBS Periyakulam 9344102671 3382 Dr. M SRINIVASAN M.B.B.S., D.orth Theni 9894620152 3392 Dr. N ETHIRAJ MBBS Theni 9842160729 3401 Dr. -
![Temple Entry Movement for Depressed Class in South Travancore [Kanyakumari] Prathika](https://docslib.b-cdn.net/cover/3132/temple-entry-movement-for-depressed-class-in-south-travancore-kanyakumari-prathika-703132.webp)
Temple Entry Movement for Depressed Class in South Travancore [Kanyakumari] Prathika
Prathika. S al. International Journal of Institutional & Industrial Research ISSN: 2456-1274, Vol. 3, Issue 1, Jan-April 2018, pp.4-7 Temple Entry Movement for Depressed Class in South Travancore [Kanyakumari] Prathika. S Ph.D. Research Scholar, Department of History and Research Centre, S.T. Hindu College, Nagercoil 629002. Abstract: The four Tamil speaking taluks of Kanyakumari Dist viz;Agasteeswaram, Thovalai, Kalkulam and Vilavancode consisted the erst while South Tavancore. Among the various religions, Hinduism is the predominant one constituting about two third of the total population. The important Hindu temples found in Kanyakumari District are at Kanyakumari, Suchindrum, Kumarakoil,Nagercoil, Thiruvattar and Padmanabhapuram. The village God like Madan,Isakki, Sasta are worshipped by the Hindus. The people of South Travancore segregated and lived on the basis of caste. The whole population could be classified as Avarnas or Caste Hindus and Savarnas or non-caste people. The Savarnas such as Brahmins, Kshatriyas, Vaishyas, and Sudras who enjoyed special powers and privileges of wealth constituted the higher castes. The Avarnas viz the Nadars, Ezhavas, Mukkuvas, Sambavars, Pulayas and numerous hill tribes were considered as the polluting castes and were looked down on and had to perform various services for the Savarnas . Avarnas were not allowed in public places, temples, and the temple roads also. Low caste people or Avarnas were considered as untouchable people. Untouchability, one of the major debilities prevailed among the lower order of the society in South Travancore caused an indelible impact on the society. Keywords: Temple Entry Movement, Depressed Class, Kanyakumari reformers against that oppressive activities. -

The Spread of Jainism in Agasteeswaram Taluk
THE SPREAD OF JAINISM IN AGASTEESWARAM TALUK K. JENIFER Research Scholar, Nesamony Memorial Christian College Marthandam, Tamil Nadu, India Dr. C. SELVARAJ Professor, Lakshmipuram College of Arts and Science Manavalakurichi, Kanyakumari, Tamil Nadu, India Article Particulars: Received: 29.03.2018 Accepted: 21.04.2018 Published: 28.04.2018 Abstract Agasteeswaram taluk is a taluk located in Kanyakumari district, Tamil Nadu, India The headquarters of the taluk is the town of Nagercoil. Agastheeswaram taluk was transferred from Thiruvananthapuram District of the Travancore-Cochin State to Kanyakumari district. The origins of Jainism can be traced back to the Indus River valley civilization of 3000 B.C. Jains believe that there were 24 great teachers. These twenty-four teachers are called Tirthankaras-people who had attained all knowledge while living (Moksha) and preached it to the people. They believe in reincarnation. From a very early date Jainism flourished in Agasteeswaram taluk. The Jains believe that their religion is as old as Vedic religion. The religion of Jains was probably founded by Parsvanath known as the twenty-third Jain Tirthankara. The Jains paid due attention to the excavation of caverns, cave temples and buildings of structural temples with sculptures of Tirthankaras and their attendants such a Yakshas and Yakshis. There are no hills except the Maruthuva Mala (Medicine hill) which is noted for the numerous medicinal herbs which grow there. Maruthuvamala attracts spiritualists seeking solace in solitude. Further, the paper focuses on the spread of Jainism within Agasteeswaram taluk. Introduction Agasteeswaram taluk has great historical and cultural antiquity. It was a centre of Hinduism. -
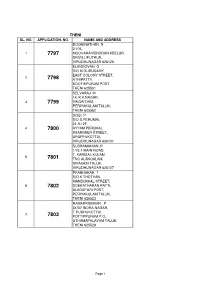
THENI APP.Pdf
THENI SL. NO. APPLICATION. NO. NAME AND ADDRESS BOOMINATHAN. R 2/105, 1 7797 MOOVARAIVENDRAN KEELUR, SRIVILLIPUTHUR, VIRUDHUNAGAR 626125 ELANGOVAN. G S/O M.GURUSAMY EAST COLONY STREET, 2 7798 ATHIPATTY, BOOTHIPURAM POST, THENI 625531 SELVARAJ. M 14, K.K.NAGAR, 3 7799 VAIGAI DAM, PERIYAKULAM TALUK, THENI 625562 GOBI. P S/O S.PERUMAL 21-9-12F, 4 7800 AYYAM PERUMAL ASARIMIER STREET, ARUPPUKOTTAI, VIRUDHUNAGAR 626101 SUBRAMANIAN .P 1/73-1 MAIN ROAD, T. KARISAL KULAM, 5 7801 TNC ALANGALAM, SIVAKASI TALUK, VIRUDHUNAGAR 626127 PRABHAKAR. T S/O K.THOTHAN MANDUKKAL STREET, 6 7802 SOKKATHARAN PATTI, ALAGAPURI POST, PERIYAKULAM TALUK, THENI 626523 RAMAKRISHNAN . P 31/B7 INDRA NAGAR, T.PUDHUKOTTAI, 7 7803 POTTIPPURAM P.O, UTHAMAPALAYAM TALUK, THENI 625528 Page 1 BASKARAN. G 2/1714. OM SANTHI NAGAR, 11TH STREET, 8 7804 ARANMANAI SALI, COLLECTRATE POST, RAMNAD 623503 SURESHKUMAR.S 119, LAKSHMIAPURAM, 9 7805 INAM KARISAL KULAM (POST), SRIVILLIPUTTUR, VIRUTHU NAGAR 626125 VIJAYASANTHI. R D/O P.RAJ 166, NORTH STREET, 10 7806 UPPUKKOTTAI, BODI TK, THENI 625534 RAMJI.A S/O P.AYYAR 5/107, NEHRUNAGAR, 11 7807 E-PUTHUKOTTAI, MURUGAMALAI NAGAR (PO), PERIYAKULAM (TK), THENI 625605 KRISHNASAMY. M 195/31, 12 7808 GANDHIPURAM STREET, VIRUDHUNAGAR 626001 SIVANESAN. M 6/585-3A, MSSM ILLAM, 13 7809 3RD CROSS STREET, LAKSHMI NAGAR, VIRUDHUNAGAR 626001 GIRI. G S/O GOVINDARAJ. I 69, NORTH KARISALKULAM, 14 7810 INAM KARISAL KULAM POST, SRIVILLIPUTTUR TALUK, VIRUDHUNAGAR 626125 PARTHASARATHY. V S/O VELUSAMY 2-3, TNH,BVANNIAMPATTY, 15 7811 VILLAKKUINAM, KARISALKULAM POST, SRIVILLIPUTHUR TALUK, VIRUDHUNAGAR 626125 Page 2 MAHARAJA.S 11, WEST STREET, MANICKPURAM, 16 7812 KAMARAJAPURAM (PO), BODI (TK), THENI 625682 PALANICHAMY.