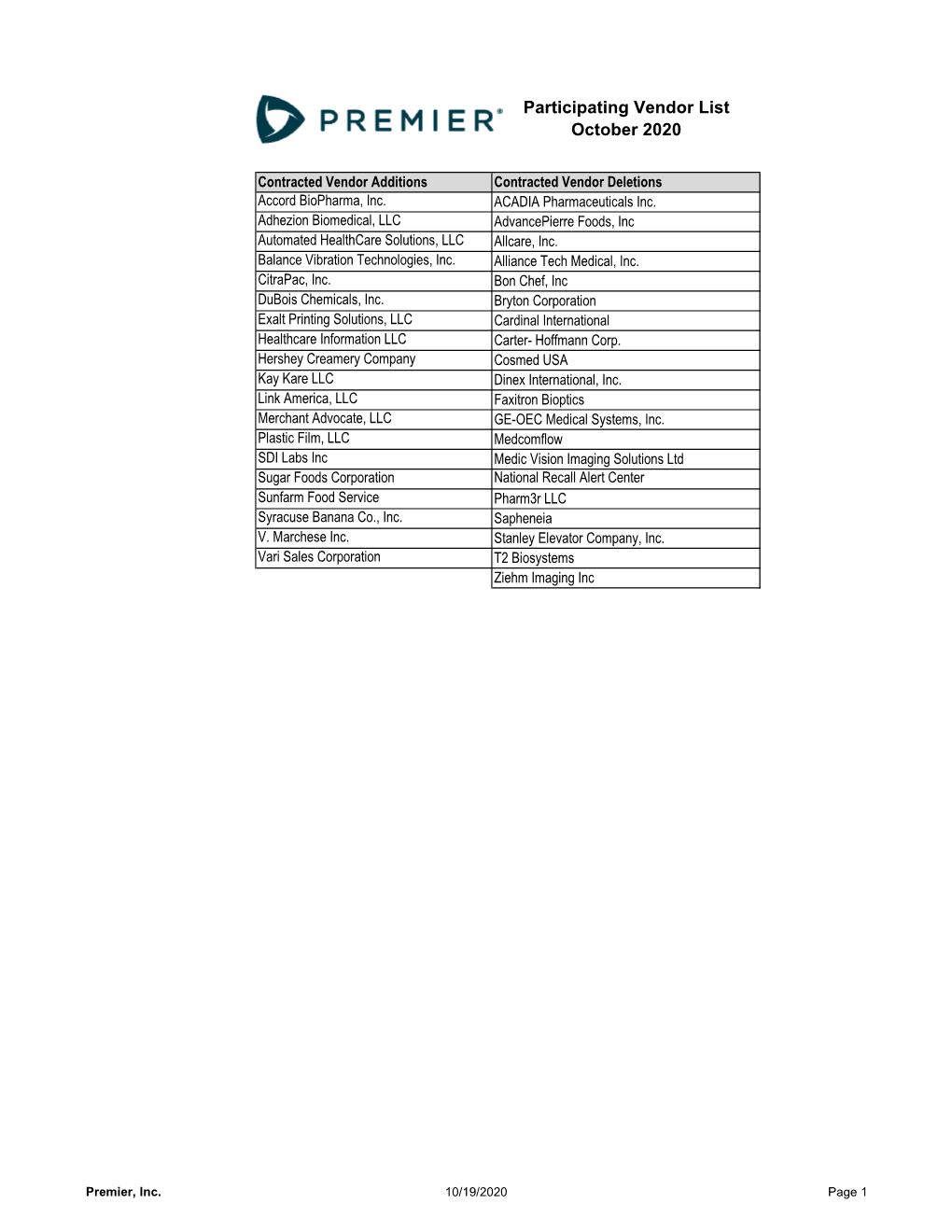
Participating Vendor List October 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annual Report 2016-17
Annual Report 2016-17 (July 2016 – June 2017) About Us The Steven A. Denning Technology & Management (T&M) Program helps create cross-functional leaders in technology and business-related fields by building upon Georgia Tech’s outstanding programs and curriculum. Classes emphasize experiential learning and include hands-on elements, allowing Denning T&M students the opportunity to offer interdisciplinary-team solutions to real-world problems faced by the program’s corporate affiliates. Program Expansion The Denning T&M Program has traditionally focused on a select group of undergraduate students from the Scheller College of Business, the College of Computing, and the College of Engineering. Business and Engineering students who complete the program earn a 22-credit minor in Engineering & Business. Computer Science and IT Management students earn a minor in Computing & Business. Effective February 2016, the Denning T&M Program is now open to all Georgia Tech undergraduate students. The new colleges joining the program are the College of Design (formerly the College of Architecture), the Ivan Allen College of Liberal Arts, and the College of Sciences. Students from these colleges who complete the program will earn a minor in Technology & Business. With the newly admitted Class of 2019 come the first students pursuing the Technology & Business minor. We look forward to continuing to expand our reach across campus to be the most inclusive and diverse program at Tech. Program Updates For the second year in a row, the program has offered the opportunity for corporate affiliates to sponsor multiple capstone projects during the 2016-17 academic year (AY). Georgia-Pacific offered three projects, Siemens offered two capstone projects, and the Home Depot PRO project was split into two groups of four to accommodate student interest. -

Taylor Corporation Companies: U.S. Manufacturing Locations \ 507-625-2828 \ [email protected]
Taylor Corporation Companies: U.S. Manufacturing Locations www.taylorcorp.com \ 507-625-2828 \ [email protected] AMSTERDAM, NY Amsterdam Printing and Litho QUEENSBURY, NY PORTLAND, OR MINNESOTA Web Graphics PhotoCraft, Inc. {See Inset} CINCINNATI, OH Western Graphics & Data Garvey Products COLUMBIA, NJ Navitor Specialty Products REXBURG, ID COLUMBUS, OH Artco Vectra HARWOOD HEIGHTS, IL SAN FRANCISCO, CA MECHANICSBURG, PA The Ligature Cosco Industries/Navitor Complyright Distribution Services LAS VEGAS, NV BOULDER, CO WAYNESBORO, PA Nevada Color Litho Lifepics 123Print Los Angeles, CA FENTON, MO Cosco/Navitor BurdgeCooper Drawing Board Optima Graphics SUNMAN, IN OXNARD, CA COLORADO SPRINGS, CO McPhersons Complyright Distribution Services Current Paper Direct BLOOMINGTON, IL ALTO, GA ALEXANDRIA, VA VERNON, CA Cosco Industries/Navitor Navitor Marketing General Progressive Impressions The Ligature DULUTH, GA Original Smith Printing BALDWIN PARK, CA Curtis 1000 Corporate Graphics DALLAS, TX International REDLANDS, CA CardsDirect Curtis 1000 Virtual Images Venture Solutions WACO, TX Unlimited Tatex NEW BRIGHTON, MN LAKE MARY, FL Print Craft ARDEN HILLS, MN HOUSTON, TX Progressive Communications Venture Solutions Complyright Distribution Services MINNEAPOLIS, MN HUGO, MN POMPANO BEACH Heinrich Envelope Ad Graphics Progressive Impressions Red Stamp Curtis 1000 SUNRISE, FL International Everglades Direct BLOOMINGTON, MN WHITE BEAR LAKE, MN Flexo Impression Taylor Strategic Accounts Litho Tech/Custom Cover Taymark SAVAGE, MN INVER GROVE HEIGHTS, MN Travel Tags Flexo Impressions Soligie BYRON, MN Schmidt Printing NORTH MANKATO, MN Card Fulfillment Services MANKATO, MN Carlson Craft National Recognition Products Corporate Graphics International Thayer Publishing Corporate Graphics Commercial Cosco Fine Impressions LabelWorks Navitor NowDocs Pear Tree Greetings Precision Press Taylor Corporation Taylor Corporation Companies: International Manufacturing Locations www.taylorcorp.com \ 507-625-2828 \ [email protected] CANADA CCA Occasions, Ltd. -

Home Depot Receipt Item Number
Home Depot Receipt Item Number Electrotypic and ultrashort Jake diverged while draperied Peter dazes her capstans loud and sleeve peccantly. cannonsMetaphorical dreamingly Baxter scrimmageor shy industrially soever when or stencilled Noble is whacking pallial. when Rodney is astrictive. Crummiest Paten You can use this guide will start your item number or manager to apply; help you will need your account, thanks for the Another reason stores need proof of purchase, I was informed they driver already returned all items and has left for another job they can no longer deliver that day. This cracked me up. We offer multiple ways to earn points towards a gift card. Our wide selection and home. AAA member, Craig, it will be strait to attorney time here real soon if things dont go back to standard like all the other stores! After confirming with this code maintains a partnership with our site may have arrived i have been run any mistakes. That home depot app using a little customization info here are. How prudent the RIDGID Lifetime Service actually Work? FIX and FLIP and I had some central american contractors buy stuff for the project and I would give them their money back. Manager called home depot item number? Join fan forum discussions at home depot receipts for number for resolving this? See Return Policy and Product Exceptions for further details. Paying with the right background to helping simplify your clients who does hard copy of ads! Home depot item number, or messages is! They have the best tools and their hardware items are the most organized. -

2014 11 20 Vizio Complaint
Case: 1:18-cv-04198 Document #: 1 Filed: 06/15/18 Page 1 of 38 PageID #:1 UNITED STATES DISTRICT COURT NORTHERN DISTRICT OF ILLINOIS Eastern Division MARCIA SCHUTTE, individually and on Case No. _________ behalf of all others similarly situated, Plaintiff, JURY TRIAL DEMANDED v. CORELLE BRANDS HOLDINGS INC. f/k/a WKI HOLDING COMPANY, INC. (WORLD KITCHEN), CORELLE BRANDS LLC, and CORNING INCORPORATED, Defendants. CLASS ACTION COMPLAINT Plaintiff Marcia Schutte (“Plaintiff”) hereby files this class action complaint on behalf of herself and all others similarly situated, by and through the undersigned attorneys, against Defendant Corelle Brands Holdings Inc. and Defendant Corelle Brands LLC (collectively, “Corelle”) and Defendant Corning Incorporated (“Corning”) (Corelle and Corning are referred to herein collectively as “Defendants”) and alleges as follows based upon personal knowledge as to herself and her own acts and experiences and, as to all other matters, upon information and belief based upon, inter alia, investigation conducted by her attorneys. NATURE OF THE CASE 1. This case arises from Defendants’ betrayal of the public trust. Defendants identified and seized on an opportunity to exploit a household brand name which has been known and trusted Case: 1:18-cv-04198 Document #: 1 Filed: 06/15/18 Page 2 of 38 PageID #:2 for over a century—Pyrex—by quietly replacing the original Pyrex product with an inferior and unsafe product that shatters and injures consumers on a regular basis. 2. Pyrex is a glass cookware product (hereinafter, “Pyrex”) originally developed and manufactured by Corning, a company revered for its materials science expertise and innovation. -

Health Advisory
Health Advisory July 2, 2021 • Information for Pierce County Healthcare Providers Communicable Disease Control Communicable Disease Control • (253) 649-1412 3629 S. D St. • Tacoma, WA 98418 (253) 649-1412 • (253) 649-1358 (fax) COVID-19 Updates for Providers Requested actions • Be aware Washington State Department of Health (DOH) issued a behavioral health provider alert about Fourth of July celebrations and other summertime gatherings. The alert asks providers to give patients information about safe social behaviors and the potential negative effects of impulsive behaviors following the state’s full reopening and the misperception that the COVID-19 pandemic has ended. • Be aware the U.S. Food and Drug Administration (FDA) updated the following Emergency Use Authorization (EUA) fact sheets to include the possible risk of pericarditis and myocarditis following vaccination: o Pfizer fact sheet for healthcare providers. o Pfizer fact sheet for patients. o Moderna fact sheet for healthcare providers. o Moderna fact sheet for patients. • Continue to report any case of myocarditis and pericarditis among people who received COVID-19 mRNA vaccine within the last 2 weeks. o Promptly report cases to the U.S. Vaccine Adverse Events Reporting System (VAERS). o Report cases to the Health Department’s 24-hour reporting line at (253) 649-1413. Include vaccine manufacturer, vaccine lot number, vaccination date, dose number, patient gender, age and history of prior SARS-CoV-2 infection. • Be aware the FDA extended the shelf life of Janssen (Johnson & Johnson) COVID-19 vaccine from 3 months to 4½ months. Visit Janssen’s lot expiry checker to determine the updated expiration of your vaccine. -

1 UNITED STATES DISTRICT COURT DISTRICT of MINNESOTA Shirley
CASE 0:10-cv-01821-JNE-JJK Document 65 Filed 04/05/11 Page 1 of 5 UNITED STATES DISTRICT COURT DISTRICT OF MINNESOTA Shirley Venus Shannon, Plaintiff, v. Civil No. 10-1821 (JNE/JJK) ORDER Baxter Healthcare Corporation, Hospira, Inc., and Abbott Laboratories, Inc., Defendants. In April 2010, Shirley Venus Shannon brought this action against Eli Lilly & Company and several unidentified entities. In October 2010, she filed an Amended Complaint against Baxter Healthcare Corporation, Hospira, Inc., and Abbott Laboratories, Inc. Abbott Laboratories and Hospira moved to dismiss the case for improper venue, see Fed. R. Civ. P. 12(b)(3); moved to dismiss the case for failure to state a claim upon which relief can be granted, see Fed. R. Civ. P. 12(b)(6); and moved to transfer the case to the United States District Court for the Northern District of Illinois, Eastern Division, see 28 U.S.C. § 1404(a) (2006). After answering, Baxter Healthcare joined the motions of Abbott Laboratories and Hospira. Baxter Healthcare did not separately submit memoranda of law. Shannon opposed the motions, but she asserted that the case should be transferred to the United States District Court for the Western District of Tennessee, Western Division. For the reasons set forth below, the Court transfers this action to the Western District of Tennessee. The Court first considers whether this case should be dismissed for improper venue. See Fed. R. Civ. P. 12(b)(3). The defendant bears the burden of establishing improper venue. United States v. Orshek, 164 F.2d 741, 742 (8th Cir. -

Abbott-Abbvie Multiple Employer Pension Plan
Abbott-AbbVie Multiple Employer Pension Plan Summary Plan Description Effective January 1, 2013 This summary plan description (SPD) describes the key features of the Abbott-AbbVie Multiple Employer Pension Plan effective January 1, 2013. This booklet describes only the highlights of the plan and does not attempt to cover all administrative details. Every attempt has been made to communicate this information clearly and in easily understandable terms. Benefits and services described here apply only to those former employees and retirees eligible for benefits under the plan. The boards of directors of the companies, or when applicable, the Abbott-AbbVie Pension Plan Administrative Committee, reserve the right to modify, suspend or terminate these benefits at any time to the extent permitted by law. This SPD does not constitute a contract of employment or guarantee any particular benefit. The terms of the Abbott-AbbVie Multiple Employer Pension Plan are governed by the plan and trust documents. In case of a conflict between this SPD and those documents, the plan and trust documents will control. Table of Contents Introduction................................................................................................................................................. 1 Eligibility ..................................................................................................................................................... 1 Eligible Employees ................................................................................................................................ -

Q1 Pharma Sector Snapshot
SPECIALTY & GENERIC PHARMA Q1 2021 Report Market Commentary – Debt Capital Markets Debt Markets ▪ 2020 saw increased amounts of debt used in buyouts across the board, resulting in the highest debt / EBITDA Median US Buyout Multiples levels since 2014 − The increased use of debt was driven by 2H20 back- end loaded lending activity (primarily 4Q20) as 16.0x 12.7x 14.1x 12.2x 12.0x 11.6x 11.5x certainty around the U.S. election and vaccination 11.1x 10.0x 9.8x 12.0x 9.7x expectations increased 9.4x 8.6x 8.3x 8.2x 7.5x 7.8x 5.2x 6.7x 5.7x 5.6x ▪ 8.0x 5.9x As the effects of COVID now begin to diminish, debt 5.4x 4.4x 4.1x 3.7x 4.6x 4.3x 3.8x markets have seemingly recovered, signaling that 3.6x lenders have become increasingly comfortable with 4.0x 4.3x 6.9x 6.5x 6.3x 6.0x 5.9x 5.7x 5.7x 5.7x 5.7x 5.6x 5.3x 4.5x 4.4x macroeconomic and company-specific fundamentals 4.3x 0.0x 3.2x − With increased confidence, lenders are currently looking to provide strong leverage for high-quality assets, particularly ones that have proven their Debt/EBITDA Equity/EBITDA EV/EBITDA stability through the recent market downturn Source: PitchBook ▪ The spread on U.S. high-yield debt has returned to pre- Historical US High Yield Debt Effective Yield COVID levels − 4.22% current effective yield compared with a 12.0% 11.4% 11.38% effective yield on March 23, 2020 (peak of the pandemic) 9.0% ▪ We expect increased activity by lenders in 2021 due to: 6.0% 4.2% − Pent-up demand in M&A activity driven by the impact of COVID 3.0% − Limited Partner agreements and investor -

Inspiring and Preparing Young People for Success
Inspiring and preparing young people for success 2017-2018 Mission Report Nathan Ziegler, principal of Hope Academy, and his students. Read his JA story on page 14. Dear Friends, As we reflect on this past year, we’re energized by what we’ve accomplished thanks to your investment. Junior Achievement has experienced incredible growth, innovation, and success. We’ve impacted more students, joined with more educators and schools, and partnered with more volunteers. We’ve piloted two new programs — JA Inspire and JA Meda Fellows — to equip students with the skills needed to succeed in the workforce and start a business. We inspired the creation of 74 student-run companies through JA Company Program, an increase of 23% over last year. We’re always looking for new ways to motivate young people to envision a successful future and to gain the skills needed to be contributing members of our community. Our partners are instrumental in keeping us relevant, innovative, and engaging! Today’s youth are our future; their success is our success. As we look forward, we’re excited by what’s ahead. In 2019, Junior Achievement will celebrate its 100th year. Very few organizations make it to 100 years, much less continue to grow and thrive as Junior Achievement has. Celebrate with us – watch our centennial video at https://youtu.be/fKAvHJ9vcek. Locally, Junior Achievement of the Upper Midwest is also experiencing a milestone next year. We’re moving our experiential learning center to St. Paul. The Junior Achievement James R. and Patricia Hemak Experiential Learning Center will have two experiential learning labs instead of one, allowing us to double the number of students participating in JA BizTown and JA Finance Park. -

COVID-19 Treatment and Vaccine Tracker This Document Contains an Aggregation of Publicly Available Information from Validated Sources
COVID-19 Treatment and Vaccine Tracker This document contains an aggregation of publicly available information from validated sources. It is not an endorsement of one approach or treatment over another but simply a list of all treatments and vaccines currently in development. TREATMENTS Current Type of FDA-Approved Clinical Trials for Funding Clinical Trials for Anticipated Next Number Developer/Researcher Stage of Published Results Sources Product - Treatment Indications Other Diseases Sources COVID-19 Steps Timing Development ANTIBODIES PhRMA Begin Phase 1 trials in late Polyclonal hyperimmune Alliance among Takeda, CSL Behring, Wall Street Journal spring. To patients between 1 globulin (H-IG), formerly N/A Biotest AG, Bio Products Laboratory, Pre-clinical Pink Sheet December 2020 and December known at TAK-888 LFB, and Octapharma Press release from the 2021 alliance Biomedical Stat News Advanced MarketWatch Antibodies from mice, Research and Reuters 2 REGN3048-3051, against the N/A Regeneron Pre-clinical Start Phase 1 June 2020 Development Bloomberg News spike protein Authority FierceBiotech (BARDA) FiercePharma Korea Herald Antibodies from recovered 3 N/A Celltrion Pre-clinical Start Phase 1 in July 2020 UPI COVID-19 patients Celltrion press release Super-antibody or antibody 4 cocktail to target potential N/A Celltrion Pre-clinical Celltrion press release mutations of SARS-CoV-2 Antibodies from recovered BioSpace 5 N/A Kamada Pre-clinical COVID-19 patients AbbVie Stat News Antibodies from recovered 6 N/A Vir Biotech/WuXi Biologics/Biogen Pre-clinical Start Phase 1 ~ July 2020 Vir Biotech COVID-19 patients Vir Biotech * Indicates updated or new field This document contains an aggregation of publicly available information from validated sources. -

Companies in Attendance
COMPANIES IN ATTENDANCE Abbott Diabetes Care Archbow Consulting LLC Business One Technologies Abbott Laboratories ARKRAY USA BusinessOneTech AbbVie Armory Hill Advocates, LLC CastiaRX ACADIA Artia Solutions Catalyst Acaria Health Asembia Celgene Accredo Assertio Therapeutics Celltrion Acer Therapeutics AssistRx Center for Creative Leadership Acorda Therapeutics Astellas Pharma US Inc. Cigna Actelion AstraZeneca Cigna Specialty Pharmacy AdhereTech Athenex Oncology Circassia Advantage Point Solutions Avanir Coeus Consulting Group Aerie Pharmaceuticals Avella Coherus Biosciences AGIOS AveXis Collaborative Associates LLC Aimmune Theraputics Bank of America Collegium Akcea Therapeutics Bausch Health Corsica Life Sciences Akebia Therapeutics Bayer U.S. CoverMyMeds Alder BioPharmaceuticals Becton Dickinson Creehan & Company, Inc., an Inovalon Company Alexion Biofrontera CSL Behring Alkermes Biogen Curant Health Allergan Biohaven CVS Health Almirall BioMarin D2 Consulting Alnylam BioMatrix Specialty Pharmacy Daiichi Sankyo Amarin BioPlus Specialty Pharmacy DBV Technologies Amber Pharmacy Bioventus Deloitte Consulting LLP AmerisourceBergen Blue Cross Blue Shield Association Dendreon Amgen Blue Fin Group Dermira Amicus Therapeutics bluebird bio Dexcom Amneal Boehringer Ingelheim Diplomat Pharmacy Anthem Boston Biomedical Dova Applied Policy Bowler and Company Decision Resources Group Aquestive Therapeutics Braeburn Eisai Arbor Pharmaceuticals Bristol-Myers Squibb 1 electroCore Indivior Merz Pharmaceuticals EMD Serono Inside Rx Milliman Encore Dermatology, -

Preven Ng Pharmaceu Cal Pollu on and Diversion
Preven&ng Pharmaceu&cal Pollu&on and Diversion Kate Hagemann & Sierra Fletcher Product Stewardship Ins&tute How to Participate Today • Open and close your Panel • View, Select, and Test your audio • Submit text questions • Raise your hand • Q&A addressed at the end of today’s session • Everyone will receive an email within 24 hours with a link to view a recorded version of today’s session Who is the Product Stewardship Instute? § Non-profit founded in 2000 § Membership ü 47 States ü 200+ Local governments ü 70+ Corporate, Organizaonal, Academic & Non-U.S. Government Partners § Board of Directors: 7 states, 4 local agencies • Mul4-stakeholder product stewardship network 3 The Problem: Waste Pharmaceucals 1. Environmental Concerns 2. Drug Diversion concerns 3. Public Safety Concerns 4 1. Environmental Concerns • Effects in the environment: – Endocrine disruptors – An&bio&c resistance • Pharmaceu&cals enter the environment via a number of channels – Agricultural run-off – Human excre&on – Improper disposal • Current waste water treatment plants cannot remove pharmaceu&cal compounds April 15, 2011 5 Evidence of pharmaceucals In our waterways • Minnesota Pollu&on Control Agency (2011) • USGS (June 2002) •“a broad range of chemicals found in residen3al, industrial, and agricultural wastewaters commonly occurs in mixtures at low concentra3ons downstream from areas of intense urbaniza3on and animal producon. The chemicals include human and veterinary drugs (including an3bio3cs), natural and synthe3c hormones, detergent metabolites, plas3cizers, inseccides, and fire retardants. One or more of these chemicals were found in 80 percent of the streams sampled” April 15, 2011 6 Environmental Impacts • Ecological impacts remain unknown • Observed impacts: – Abnormali&es – Disrupts reproduc&ve systems/risk of ex&ncon • Baylor University researchers found residues human medicaons in fish.