Rajni Varma Govindjee in Botany, UIUC (1961)
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Secure Schools Notice
EMMETT INDEPENDENT SCHOOL DISTRICT #221 Wayne Rush, Superintendent KNOW YOUR TERMINOLOGY For Securing Our Schools Using the same Terminology throughout the district is critical in situations that impact the safety of students and staff. Three plain language terms are used by schools, police, and members of the media during such events. “Lockdown” “Hold and Secure” “Shelter in Place” "Lockdown" is used when there is a major immediate threat of serious violence inside the school or on school property. Classroom doors will be locked and students are moved to a safe area in the classroom. All movement in and out of the school and within the school is restricted. Outside doors are to be locked, except in the case where the threat is already in the building. "Hold and Secure" is used to secure the school due to an ongoing situation outside the school that requires all persons to remain in the building. Examples may include a bank robbery or domestic situation near a school. The school continues to function normally, with the exterior doors locked until the situation is resolved. Staff member posted near each entrance to ensure no one leaves the building. All movement in and out of the school is restricted. Notification of situation may come from local law enforcement or school employees. "Shelter in Place" is used for an environmental, or weather related situation, where it is necessary to keep all occupants within the school for their protection. This response may be recommended by Police, Fire Department, EMS, etc. Examples may include chemical spills, blackouts, explosions, or extreme weather conditions. -

Smoke Communication Strategy and Appendices 2007
A W F C G S M O K E E D U C A T I O N C Smoke Education Communication Strategy O M v.2007 M U N I C A T I O N S Approved: Date: T R A _____________________________ __________________ T AWFCG Chair E G Y v.2007 AWFCG Smoke Education Communications Strategy Page 2 of 11 02/26/07 Table of Contents Section Page / Appendix Purpose 3 Background 3 Communication Goals 3 General Audiences 3 Strategy 4 Tactics 5 Success 6 Tools and Products 6 Target Audiences 6 Target Media 8 Appendices 11 News Release A Key Messages B Talking Points C Public Service Announcement D Poster E Flyer F Web Site Plan G Display Panel 1 H Display Panel 2 I v.2007 AWFCG Smoke Education Communications Strategy Page 3 of 11 02/26/07 Purpose To provide members of the Alaska Wildland Fire Coordinating Group (AWFCG) with a communication strategy to engage the public in smoke information from wildland fires which include prescribe fires, fire use and wildfires, occurring in the State of Alaska. Background The increase in smoke throughout Alaska during the 2004 and 2005 fire seasons hampered fire suppression operations, aviation operations, motor vehicle operations, tourism and recreation. This strategy provides a collective approach to informing the public about smoke-related issues. Communication Goals · Develop a set of key messages to be used by AWFCG member organizations in order to project one voice in a unified effort regarding smoke issues and mitigation measures. · Provide focused communication products that support the communication goals of this strategy. -

Public Information Statement Spotter Reports National Weather Service Burlington Vt 1000 Am Est Thu Feb 28 2008
PUBLIC INFORMATION STATEMENT SPOTTER REPORTS NATIONAL WEATHER SERVICE BURLINGTON VT 1000 AM EST THU FEB 28 2008 THE FOLLOWING ARE UNOFFICIAL 2 DAY SNOWFALL REPORTS FROM THE STORM THAT AFFECTED OUR REGION FROM EARLY TUESDAY MORNING ON FEBRUARY 26TH THROUGH EARLY THURSDAY MORNING FEBRUARY 28TH. APPRECIATION IS EXTENDED TO HIGHWAY DEPARTMENTS...COOPERATIVE OBSERVERS...SKYWARN SPOTTERS AND MEDIA FOR THESE REPORTS. THIS SUMMARY IS ALSO AVAILABLE ON OUR HOME PAGE AT WEATHER.GOV/BURLINGTON ********************STORM TOTAL SNOWFALL******************** LOCATION STORM TOTAL TIME/DATE COMMENTS SNOWFALL OF (INCHES) MEASUREMENT NEW YORK ...CLINTON COUNTY... ELLENBURG DEPOT 10.5 700 AM 2/28 COOP PLATTSBURGH 8.0 700 PM 2/27 HAM PERU 5.8 800 AM 2/28 SPOTTER ...ESSEX COUNTY... NEWCOMB 7.8 900 AM 2/28 COOP ...FRANKLIN COUNTY... TUPPER LAKE SUNMOUNT 9.0 700 AM 2/28 COOP MALONE 5.5 700 AM 2/28 COOP ...ST. LAWRENCE COUNTY... EDWARDS 5.0 700 PM 2/27 HAM CANTON 4 SE 4.0 700 AM 2/28 COOP NORFOLK 0.4 940 AM 2/28 VERMONT ...ADDISON COUNTY... SOUTH LINCOLN 5.9 700 AM 2/28 COOP ...CALEDONIA COUNTY... SUTTON 2 NE 10.0 700 AM 2/28 COOP ...CHITTENDEN COUNTY... NORTH UNDERHILL 13.0 700 AM 2/28 NWS EMPLOYEE SOUTH BURLINGTON 11.6 700 AM 2/28 NWS OFFICE HANKSVILLE 10.6 700 AM 2/28 COOP ESSEX JUNCTION 1 N 9.6 700 AM 2/28 COOP JERICHO CENTER 3 ESE 9.6 650 AM 2/28 NWS EMPLOYEE SOUTH BURLINGTON 7.5 700 AM 2/28 NWS EMPLOYEE ...FRANKLIN COUNTY.. -

Nevada Broadcasters Association Sober Moms Total Dollar Return
Sober Moms Total Dollar Return and Spots Aired For March 2016 Monthly Investment : $5000.00 Region Spots Aired Region Total Estimated Value Southern Radio 692 Southern Radio $69,200.00 Southern Television 321 Southern Television $53,025.00 Northern and Rural Radio 527 Northern and Rural Radio $39,525.00 Northern and Rural Television 960 Northern and Rural Television $151,800.00 Monthly Spot Total 2,500 Monthly Value Total $313,550.00 Campaign Spot Total 8,663 Campaign Value Total $1,095,120.00 Monthly Return on Investment 62:1 Total Return on Investment 54:1 Spots Aired Day Parts Spots Aired 35% 42% 6am to 7pm 6am to 7pm 871 7pm to 12am 573 7pm to 12am 12am to 6am 1056 23% 12am to 6am Station Frequency Format Spots Total Value* 6a-7p 7p-12a 12a-6a KBAD 920 AM Sports 9 $900.00 3 3 3 KCYE 102.7 FM Coyote Country 10 $1,000.00 0 0 10 KDWN 720 AM News/Talk 10 $1,000.00 0 0 10 KENO 1460 AM Sports 9 $900.00 3 3 3 KISF 103.5 FM Regional Mexican 23 $2,300.00 5 8 10 KJUL 104.7 FM Adult Standards 41 $4,100.00 4 27 10 KKLZ 96.3 FM Classic Rock 10 $1,000.00 0 0 10 KLAV 1230 AM Talk/Information 9 $900.00 3 3 3 KLSQ 870 AM Spanish Oldies/Talk 21 $2,100.00 10 2 9 KLUC 98.5 FM Contemporary Hits 42 $4,200.00 0 0 42 KMXB 94.1 FM Modern Adult Contemporary 44 $4,400.00 0 3 41 KMZQ 670 AM News/Talk 70 $7,000.00 35 15 20 KOAS 105.7 FM Jazz 10 $1,000.00 0 0 10 KOMP 92.3 FM Rock 8 $800.00 2 2 4 KPLV 93.1 FM Oldies 6 $600.00 1 0 5 KQLL 102.3 FM /1280 AM Oldies 24 $2,400.00 3 5 16 KQRT 105.1 FM Mexican Regional Music 36 $3,600.00 19 4 13 KRGT 99.3 FM Spanish Urban -

Radio Stations
Date Contacted Comments RA_Call EMail FirstName Bluegrass(from Missy) James H. Bluegrass(from Missy) Joe Bluegrass(from Missy) James H. Sent dpk thru Airplay Direct [email protected] 2/9/2014 Bluegrass(from Missy) m Tom Sent dpk thru Airplay Direct cindy@kneedeepi 2/9/2014 Bluegrass(from Missy) nbluegrass.com Cindy Sent dpk thru Airplay Direct drdobro@mindspri 2/9/2014 Bluegrass(from Missy) ng.com Lawrence E. Sent dpk thru Airplay Direct georgemcknight@ 2/9/2014 Bluegrass(from Missy) telus.net George Sent dpk thru Airplay Direct greatstuffradio@y 2/9/2014 Bluegrass(from Missy) ahoo.com Gene Sent dpk thru Airplay Direct jadonchris@netco 2/9/2014 Bluegrass(from Missy) mmander.com Jadon Sent dpk thru Airplay Direct roy@mainstreetbl 2/9/2014 Bluegrass(from Missy) uegrass.com Roy From Americana Music Association reporting stations list ACOUSTIC CAFE Rob From Americana Music Association reporting stations list ALTVILLE Vicki From Americana Music Association reporting stations list Country Bear Stan From Americana Music Association reporting stations list Current 89.3 David From Americana Music Association reporting stations list Farm Fresh Radio Chip From Americana Music Association reporting stations list Folk Alley - WKSU Linda From Americana Music Association reporting stations list FolkScene Roz Sending physical copy 2/2014 per his arthu2go@yahoo. facebook request. Bluegrass(from Missy) 105.9 Bishop FM co.uk Terry Sent dpk thru Airplay Direct lindsay@ozemail. 2/9/2014 Bluegrass(from Missy) 2RRR com.au Lindsay Sent dpk thru Airplay Direct tony.lake@amtac. 2/9/2014 Bluegrass(from Missy) 400R net Tony Sent dpk thru Airplay Direct bluemoon@bluegr 2/9/2014 Bluegrass(from Missy) ACTV-4 asstracks.net Jon C. -

Muttern / Bearing Nuts Artikelnr
b 1 G h d3 d1 G D B Muttern / Bearing nuts Artikelnr. Gewinde-Ø Dimensionen / Principal D imensions Gewicht kg U-Scheibe verzahnt Montageschraube Bearing No Thread-Ø B D d1 d2 b h G1 d3 Weight kg Washer, toothed Assembly Bolt Serie KM / Series KM KM 20 M 100x2 18 130 120 10 4 0,7 MB 20 KM 20 H M 100x2 18 130 120 10 4 M 6 112 0,67 MB 20 AM 6x40 KM 20 HS M 100x2 18 130 120 10 4 M 6 112 0,75 MB 20 AM 6x40 KM 21 M 105x2 18 140 126 12 5 0,85 MB 21 KM 21 H M 105x2 18 140 126 12 5 M 6 117 0,82 MB 21 AM 6x40 KM 21 HS M 105x2 18 140 126 12 5 M 6 117 0,9 MB 21 AM 6x40 KM 22 M 110x2 19 145 133 12 5 0,97 MB 22 KM 22 H M 110x2 19 145 133 12 5 M 6 122 0,94 MB 22 AM 6x40 KM 22 HS M 110x2 19 145 133 12 5 M 6 122 1,01 MB 22 AM 6x40 KM 23 M 115x2 19 150 137 12 5 1,01 MB 23 KM 23 H M 115x2 19 150 137 12 5 M 6 127 0,99 MB 23 AM 6x40 KM 23 HS M 115x2 19 150 137 12 5 M 6 127 1,06 MB 23 AM 6x40 KML 24 M 120x2 20 145 135 12 5 0,78 MBL 24 KM 24 M 120x2 20 155 138 12 5 1,08 MB 24 KM 24 H M 120x2 20 155 138 12 5 M 8 134 1,03 MB 24 AM 8x45 KM 24 HS M 120x2 20 155 138 12 5 M 8 134 1,16 MB 24 AM 8x45 KM 25 M 125x2 21 160 148 12 5 1,19 MB 25 KM 25 H M 125x2 21 160 148 12 5 M 8 139 1,14 MB 25 AM 8x45 KM 25 HS M 125x2 21 160 148 12 5 M 8 139 1,27 MB 25 AM 8x45 KML 26 M 130x2 21 155 145 12 5 0,88 MBL 26 KM 26 M 130x2 21 165 149 12 5 1,25 MB 26 KM 26 H M 130x2 21 165 149 12 5 M 8 144 1,2 MB 26 AM 8x45 KM 26 HS M 130x2 21 165 149 12 5 M 8 144 1,33 MB 26 AM 8x45 KM 27 M 135x2 22 175 160 14 6 1,55 MB 27 KM 27 H M 135x2 22 175 160 14 6 M 8 149 1,5 MB 27 AM 8x45 KM 27 HS M -
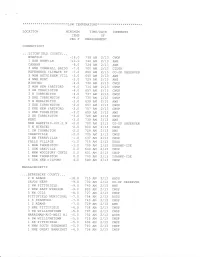
Low Temperatures
* * * * * * * * )k * * * * * * * * * * * * )k *Low TEMpER;\TURE * * * * * * * * * * * * * * * * * * * * * * * LOCAT]ON MINIMUM T]ME/DATE COMMENTS TEMP OF DEG F MEASUREMENT CONNECTICUT . ..L]TCHFIELD COUNTY., . NOREOLK _18.0 738 AM 2/73 CWOP _10 1 ESE NORFC LK . O 740 AM 2 /13 AWS _B. CANAAN O 724 AM 2/13 AWS 3 ENE CORNI^ALL BRIDG --l .0 7OO AM 2/L3 CTDEP REFERENCE CLIMATE ST -7.0 8OO AM 2/73 co-oP OBSERVER 3 WSW BETHIEHEM V]LL -5.0 645 AM 2 /13 AWS 4 NNE KENT _5.0 7 29 a]4 2/73 AWS W]NSTED _4.0 736 AM 2/13 cwoP 2 WSW NEW FARTFORD *4.0 714 AM 2/L3 cwoP 3 SW TORRINGTON _4.A 557 AM 2/73 CWOP 3 E TORR]NCTON _4.A 737 AM 2/73 CWOP 3 ENE TORR]NGTON _4.0 730 AM 2/13 CWOP 5 W BURLINCTON -3.0 539 AM 2/L3 AWS 3 ESE TORRINGTON _3.0 557 AM 2/L3 CWOP 2 ENE NEW FARTFORD _3.0 757 AM 2/73 CWOP 1 ENE THOMTSTON _3.0 659 AM 2/73 AWS 2 SE TORR]NGTON _3.0 7 26 Ar4 2/73 CWOP KENT _3.0 739 AM 2/13 AWS NEW HARTFOFD_1M].E N _2.0 7OO AM 2/13 co-oP OBSERVER 5 N W]NSTET _2.A 8OO AM 2/13 cwoP 1 SW THOMASTON _2.A 729 AM 2/73 AWS TERRYVILLE _2.0 705 AM 2/L3 CWOP 2 NW TERRY\ILLE _1.0 637 AM 2/L3 CWOP FALLS VILL}GE _1.0 715 AM 2/13 usGS 1 WSW THOM}STON _1.0 7OO AM 2/13 USARMY-COE 1 SSW OAKVILLE O.O 640 AM 2/73 CWOP 1 WNW WOODEURY CENTE O.O 601 AM 2/13 cwoP 1 NNE THOM}STON O.O 7OO AM 2/L3 USARMY_COE 5 SSW NEW NTLFORD 4.0 549 AM 2/L3 CWOP MASSACHUSETTS .BERKSHTRE COUNTY.. -

Pns121317.Pdf
PNSGYX MEZ007>009-012>014-018>028-NHZ001>015-140408- Public Information Statement Spotter Reports National Weather Service Gray ME 1108 AM EST Wed Dec 13 2017 The following are unoffical observations taken during the past 30 hours for the storm that has been affecting our region. Appreciation is extended to highway departments...cooperative observers...Skywarn spotters and media for these reports. This summary is also available on our home page at weather.gov/gray ********************STORM TOTAL SNOWFALL******************** LOCATION STORM TOTAL TIME/DATE COMMENTS SNOWFALL OF /INCHES/ MEASUREMENT MAINE ...Androscoggin County... Auburn 6.0 400 PM 12/12 Amateur Radio 3 E Turner 4.0 700 AM 12/13 CoCoRaHS 1 NW Auburn 3.8 622 PM 12/12 TRAINED SPOTTER 1 W Leeds 3.2 510 AM 12/13 CoCoRaHS Durham 3.1 444 PM 12/12 Co-Op Observer 2 E Lewiston 3.0 449 PM 12/12 TRAINED SPOTTER Poland 3.0 1100 PM 12/12 TRAINED SPOTTER Turner 2.5 700 AM 12/13 Co-Op Observer 1 ENE Lisbon Falls 2.0 453 PM 12/12 TRAINED SPOTTER ...Cumberland County... 6 SSW Naples 5.1 930 AM 12/13 CoCoRaHS 6 S Naples 5.0 408 PM 12/12 TRAINED SPOTTER 1 NE South Portland 4.0 1247 PM 12/12 Long Island 2 ENE North Windham 3.9 744 AM 12/13 TRAINED SPOTTER 1 ENE South Windham 3.5 743 AM 12/13 TRAINED SPOTTER North Yarmouth 3.5 420 PM 12/12 Amateur Radio New Gloucester 3.0 400 PM 12/12 Amateur Radio Cumberland Center 2.9 301 PM 12/12 NWS Employee 3 N Westbrook 2.8 700 AM 12/13 CoCoRaHS 2 SSE New Gloucester 2.8 700 AM 12/13 CoCoRaHS Gray NWS Office 2.6 315 PM 12/12 NWS Office 10 SW Naples 2.5 1040 AM 12/12 Amateur Radio Portland Jetport 2.3 807 PM 12/12 ASOS Harrison 2.3 900 AM 12/13 CoCoRaHS NWS Gray Office 2.3 700 AM 12/13 Co-Op Observer 3 SSE Gorham 2.3 109 PM 12/12 TRAINED SPOTTER Portland 2.0 1105 AM 12/12 Amateur Radio 3 WNW Falmouth 2.0 700 AM 12/13 CoCoRaHS 3 E Standish 1.9 1040 AM 12/12 TRAINED SPOTTER Westbrook 1.8 1023 AM 12/12 NWS Employee Portland - N Deering 1.8 416 PM 12/12 NWS Employee 3 WSW Falmouth 1.8 700 AM 12/13 CoCoRaHS 1 SSW Freeport 1.5 600 AM 12/13 CoCoRaHS ...Franklin County.. -

NOAA Extreme Weather Information(1)
To download the latest updated version of STATE INFORMATION this sheet: www.ncddc.noaa.gov/NEWIS Hawaii Emergency Management Agency (http://dod.hawaii.gov/hiema) .................................................. 808-733-4300 Ready.Gov.....................................................................................................................................www.ready.gov/hawaii Official State Website.................................................................................................................https://portal.ehawaii.gov Governor’s Office (http://governor.hawaii.gov) ........................................................................................ 808-586-0034 Attorney General (http://ag.hawaii.gov) .................................................................................................... 808-586-1500 Hawaii Tourism Authority (www.hawaiitourismauthority.org) ................................................................. 808-973-2255 Department of Agriculture (https://hdoa.hawaii.gov) .................................................................................808-973-9560 Department of Commerce and Consumer Affairs (https://cca.hawaii.gov) ................................................808-587-3222 Department of Defense (https://dod.hawaii.gov) ........................................................................................808-733-4246 Department of Public Safety (https://dps.hawaii.gov) ................................................................................808-587-1288 -

Listagem De Associados - Sem Filtro Por Palavra Por Agert - Associao Gacha De Emissoras De Rio E Televis
Listagem de Associados - Sem Filtro por Palavra Por Agert - Associao Gacha de Emissoras de Rio e Televis www.agert.org.br RÁDIO AGUDO FM AGUDO AV. CONCÓRDIA, 1480 Fone: (55)3265-1112 FM - 90.1 MHz Potencia: 2,5 KW Fundação: 14/07/1999 RÁDIO ALEGRETE AM ALEGRETE PRAÇA OSVALDO ARANHA, 39 Fone: (55)3422-1600 AM - 590 KHz Potencia: 5 KW Fundação: 07/02/1947 RÁDIO MINUANO FM (ALEGRETE) ALEGRETE RUA DOS ANDRADAS, 186 Fone: (55)3422-3788 FM - 97.5 MHz Potencia: B2 Fundação: 20/06/1986 RÁDIO NATIVA FM (ALEGRETE) ALEGRETE RUA DOS ANDRADAS, 606 SALA 04 - 3º ANDAR Fone: (55)3422-2336 FM - 105.9 MHz Potencia: B1 Fundação: 18/11/1982 RÁDIO SÃO JOSÉ FM AMARAL FERRADOR AV. CORONEL AMARAL FERRADOR, 494 Fone: (51)3670-1040 FM - 92.3 MHz Potencia: C Fundação: 19/03/2009 RÁDIO SOLARIS 101.7 FM ANTÔNIO PRADO AV. WALDOMIRO BOCCHESE, 872 Fone: (54)3293-1110 FM - 101.7 MHz Potencia: C Fundação: 26/05/1988 RÁDIO ARATIBA AM ARATIBA RUA XV DE NOVEMBRO, 336 Fone: (54)3376-1138 AM - 900 KHz Potencia: 2,5 KW Fundação: 16/06/1959 RÁDIO DIFUSORA 106.3 FM (ARROIO GRANDE) ARROIO GRANDE RUA JOSÉ BONIFÁCIO, 41 Fone: (53)3262-1008 FM - 106,3 MHz Potencia: Fundação: 09/02/1980 RÁDIO CULTURA DE ARVOREZINHA AM ARVOREZINHA AV. BARÃO DO TRIUNFO, 584 2º ANDAR Fone: (51)3772-2129 AM - 1450 KHz Potencia: Fundação: 20/09/1979 page 1 / 37 Listagem de Associados - Sem Filtro por Palavra Por Agert - Associao Gacha de Emissoras de Rio e Televis www.agert.org.br RÁDIO CULTURA DE ARVOREZINHA FM ARVOREZINHA AV. -

TAMPA BAY RAYS (43-29) at SEATTLE MARINERS (37-36) LH Shane Mcclanahan (2-2, 4.42) Vs
Press Box Documents › bit.ly/3f0l9jz TAMPA BAY RAYS (43-29) at SEATTLE MARINERS (37-36) LH Shane McClanahan (2-2, 4.42) vs. LH Marco Gonzales (1-4, 5.44) Sunday, June 20, 2021 First Pitch: 4:10 p.m. Location: T-Mobile Park TV: Bally Sports Sun Radio: WDAE 95.3 FM, WMGG 1470 AM (Sp.) Game No.: 73 (43-29) Road Game No.: 40 (24-15) All-Time Game No.: 3,695 (1769-1925) All-Time Road Game No.: 1,850 (809-1040) PRONUNCIATION GUIDE To hear Rays coaches and players pronouncing their names in their own voices, visit raysbaseball.com/pronunciation. UPCOMING PROBABLE PITCHERS & BROADCAST SCHEDULE Upcoming Games Time (ET) Probable Starting Pitchers (Rays vs. Opp.) TV & Radio Tues., 6/22 vs. BOS 7:10 p.m. TBD vs. LH Eduardo Rodriguez (5-4, 6.21) Bally Sports Sun, WDAE 95.3 FM/620 AM, WGES 680 AM (Sp.) Wed., 6/23 vs. BOS 7:10 p.m. LH Rich Hill (5-2, 3.64) vs. RH Garrett Richards (4-4, 4.36) Bally Sports Sun, WDAE 95.3 FM/620 AM, WGES 680 AM (Sp.) Thurs., 6/24 vs. BOS 7:10 p.m. TBD vs. RH Nick Pivetta (6-3, 4.36) Bally Sports Sun, WDAE 95.3 FM/620 AM, WGES 680 AM (Sp.) RAY MATTER—The Rays have lost a season-high five consecutive games, runs in the 8th lead the majors, 33 runs in the 9th rank 2nd behind the their longest losing streak since July 29–Aug 2, 2020 (5)…they would need Astros (34) and 18 runs in extras are tied for 2nd behind the Reds (21). -

Sh Bell Chicago Facility
955441 2019 S.H. BELL CHICAGO FACILITY Community Involvement Plan COMMUNITY INVOLVEMENT PLAN S.H. BELL CHICAGO FACILITY SITE TABLEOFCONTENTS Introduction 1 Purpose of this CIP, EPA's community outreach objects and brief site history. 2 Community Concerns and Questions Community members concerns, questions and comments. 3 Community Involvement Goals and Activities Goals, activities and timeline to keep residents and local officials informed and involved. 4 The Community Composition of the city of Chicago East Side Neighborhood. 5 The Site Description and history of activities. APPENDICES appendix a Definition of key words, initials and acronyms (words are in bold throughout the document). Places where community members can find more appendix b information about the site and possible meeting locations. appendix c List of federal, state and local agencies and interested parties appendix d EPA’s step-by-step process to determine the best way to clean up a contaminated site and opportunities for community involvement. appendix E Environmental Justice COMMUNITY INVOLVEMENT PLAN S.H. BELL CHICAGO FACILITY SITE INTRODUCTION Purpose of this CIP and community outreach objectives. The U.S. Environmental Protection This CIP was prepared to support Agency prepared this Community environmental and cleanup activities Involvement Plan to inform, engage near the S.H. Bell Co. Chicago facility. and support the community near the We used several information sources to S.H. Bell Company facility located in develop this plan, including research, Chicago, Illinois. Our community discussions with community members involvement effort is committed to and information gathered at community promoting effective and meaningful interviews. We conducted 26 in-person communication between the public interviews and one telephone interview and the Agency.