Cardiovascular Effects of Urocortin-2: Pathophysiological Mechanisms and Therapeutic Potential
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Corticotropin-Releasing Hormone Physiology
European Journal of Endocrinology (2006) 155 S71–S76 ISSN 0804-4643 Corticotropin-releasing hormone physiology Joseph A Majzoub Division of Endocrinology, Children’s Hospital Boston, Thomas Morgan Rotch Professor of Pediatrics, Harvard Medical School, 300 Longwood Avenue, Boston, Massachusetts 02115, USA (Correspondence should be addressed to J A Majzoub; Email: [email protected]) Abstract Corticotropin-releasing hormone (CRH), also known as corticotropin-releasing factor, is a highly conserved peptide hormone comprising 41 amino acid residues. Its name derives from its role in the anterior pituitary, where it mediates the release of corticotropin (ACTH) leading to the release of adrenocortical steroids. CRH is the major hypothalamic activator of the hypothalamic–pituitary– adrenal (HPA)axis. Major functions of the HPAinclude: (i) influencing fetal development of major organ systems including lung, liver, and gut, (ii) metabolic functions, including the maintenance of normal blood glucose levels during the fasting state via glycogenolysis and gluconeogenesis, (iii) modulation of immune function, and (iv) maintenance of cardiovascular tone. In addition, CRH, acting both directly and via the HPA, has a role in regulating several neuroendocrine functions including behavior, food intake, reproduction, growth, immune function, and autonomic function. CRH has been localized to the paraventricular nucleus (PVN) of the hypothalamus, which projects to the median eminence and other hypothalamic and midbrain targets. The CRH gene is composed of two exons. The CRH promoter contains a cAMP-response element, and the intron contains a restrictive element-1/neuron restrictive silencing element (RE-1/NRSE) sequence. Recently, a family of CRH-related peptides, termed the urocortins, has been identified. -

Expression of Urocortin and Corticotropin-Releasing Hormone Receptors in the Horse Thyroid Gland
Cell Tissue Res DOI 10.1007/s00441-012-1450-4 REGULAR ARTICLE Expression of urocortin and corticotropin-releasing hormone receptors in the horse thyroid gland Caterina Squillacioti & Adriana De Luca & Sabrina Alì & Salvatore Paino & Giovanna Liguori & Nicola Mirabella Received: 11 January 2012 /Accepted: 3 May 2012 # Springer-Verlag 2012 Abstract Urocortin (UCN) is a 40-amino-acid peptide and UCN, CRHR1 and CRHR2 and that UCN plays a role in a member of the corticotropin-releasing hormone (CRH) the regulation of thyroid physiological functions through a family, which includes CRH, urotensin I, sauvagine, paracrine mechanism. UCN2 and UCN3. The biological actions of CRH family peptides are mediated via two types of G-protein-coupled Keywords Follicular cells . C-cells . RT-PCR . receptors, namely CRH type 1 receptor (CRHR1) and CRH Immunohistochemistry . Horse type 2 receptor (CRHR2). The biological effects of these peptides are mediated and modulated not only by CRH receptors but also via a highly conserved CRH-binding Introduction protein (CRHBP). Our aim was to investigate the expression of UCN, CRHR1, CRHR2 and CRHBP by immunohisto- Urocortin (UCN) is a peptide of 40 amino acids and is a chemistry, Western blot and reverse transcription with the member of the corticotropin-releasing hormone (CRH) fam- polymerase chain reaction (RT-PCR) in the horse thyroid ily, which includes CRH, urotensin I, sauvagine, UCN2 and gland. The results showed that UCN, CRHR1 and CRHR2 UCN3. Vaughan et al. (1995) were the first to identify UCN, were expressed in the thyroid gland, whereas CRHBP was which exhibits 45% homology to CRH (Latchman 2002; not expressed. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Searching for Novel Peptide Hormones in the Human Genome Olivier Mirabeau
Searching for novel peptide hormones in the human genome Olivier Mirabeau To cite this version: Olivier Mirabeau. Searching for novel peptide hormones in the human genome. Life Sciences [q-bio]. Université Montpellier II - Sciences et Techniques du Languedoc, 2008. English. tel-00340710 HAL Id: tel-00340710 https://tel.archives-ouvertes.fr/tel-00340710 Submitted on 21 Nov 2008 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITE MONTPELLIER II SCIENCES ET TECHNIQUES DU LANGUEDOC THESE pour obtenir le grade de DOCTEUR DE L'UNIVERSITE MONTPELLIER II Discipline : Biologie Informatique Ecole Doctorale : Sciences chimiques et biologiques pour la santé Formation doctorale : Biologie-Santé Recherche de nouvelles hormones peptidiques codées par le génome humain par Olivier Mirabeau présentée et soutenue publiquement le 30 janvier 2008 JURY M. Hubert Vaudry Rapporteur M. Jean-Philippe Vert Rapporteur Mme Nadia Rosenthal Examinatrice M. Jean Martinez Président M. Olivier Gascuel Directeur M. Cornelius Gross Examinateur Résumé Résumé Cette thèse porte sur la découverte de gènes humains non caractérisés codant pour des précurseurs à hormones peptidiques. Les hormones peptidiques (PH) ont un rôle important dans la plupart des processus physiologiques du corps humain. -
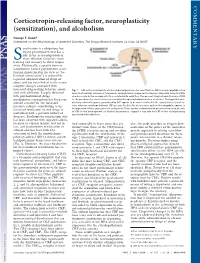
Corticotropin-Releasing Factor, Neuroplasticity (Sensitization), and Alcoholism
COMMENTARY Corticotropin-releasing factor, neuroplasticity (sensitization), and alcoholism George F. Koob* Committee on the Neurobiology of Addictive Disorders, The Scripps Research Institute, La Jolla, CA 92037 ensitization is a ubiquitous bio- logical phenomenon that has a role in the neuroadaptation of many different functions, from Slearning and memory to stress respon- sivity. Historically, a specific form of sensitization termed psychomotor sensi- tization (mistermed in my view as ‘‘be- havioral sensitization’’) is induced by repeated administration of drugs of abuse and has been linked to the neuro- adaptive changes associated with increased drug-seeking behavior associ- Fig. 1. CNS actions relevant to alcohol-induced psychomotor sensitization. CRF is a neuropeptide in the ated with addiction. Largely observed brain that controls autonomic, hormonal, and behavioral responses to stressors. New data show that CRF with psychostimulant drugs, also has a role in the neuroplasticity associated with addiction. The present study extends the role of CRF psychomotor sensitization has been con- to the psychomotor sensitization associated with repeated administration of alcohol. The hypothalamic- sidered a model for the increased pituitary-adrenal responses produced by CRF appear to be more involved in the acquisition of sensitiza- incentive salience contributing to the tion, whereas extrahypothalamic CRF systems, likely to be in structures such as the amygdala, appear to increased motivation to seek drugs in be important for the expression of sensitization. These results, combined with previous studies on the role of CRF in the development of alcohol dependence, suggest a key role for CRF in the neuroplasticity individuals with a previous history of associated with addiction. -

UCN2 (NM 033199) Human Tagged ORF Clone – RC201333 | Origene
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for RC201333 UCN2 (NM_033199) Human Tagged ORF Clone Product data: Product Type: Expression Plasmids Product Name: UCN2 (NM_033199) Human Tagged ORF Clone Tag: Myc-DDK Symbol: UCN2 Synonyms: SRP; UCN-II; UCNI; UR; URP Vector: pCMV6-Entry (PS100001) E. coli Selection: Kanamycin (25 ug/mL) Cell Selection: Neomycin ORF Nucleotide >RC201333 ORF sequence Sequence: Red=Cloning site Blue=ORF Green=Tags(s) TTTTGTAATACGACTCACTATAGGGCGGCCGGGAATTCGTCGACTGGATCCGGTACCGAGGAGATCTGCC GCCGCGATCGCC ATGACCAGGTGTGCTCTGCTGTTGCTGATGGTCCTGATGTTGGGCAGAGTCCTGGTTGTCCCAGTGACCC CTATCCCAACCTTCCAGCTCCGCCCTCAGAATTCTCCCCAGACCACTCCCCGACCTGCGGCCTCAGAGAG CCCCTCAGCTGCTCCCACATGGCCGTGGGCTGCCCAGAGCCACTGCAGCCCCACCCGCCACCCTGGCTCG CGCATTGTCCTATCGCTGGATGTCCCCATCGGCCTCTTGCAGATCTTACTGGAGCAAGCCCGGGCCAGGG CTGCCAGGGAGCAGGCCACCACCAACGCCCGCATCCTGGCCCGTGTCGGCCACTGC ACGCGTACGCGGCCGCTCGAGCAGAAACTCATCTCAGAAGAGGATCTGGCAGCAAATGATATCCTGGATT ACAAGGATGACGACGATAAGGTTTAA Protein Sequence: >RC201333 protein sequence Red=Cloning site Green=Tags(s) MTRCALLLLMVLMLGRVLVVPVTPIPTFQLRPQNSPQTTPRPAASESPSAAPTWPWAAQSHCSPTRHPGS RIVLSLDVPIGLLQILLEQARARAAREQATTNARILARVGHC TRTRPLEQKLISEEDLAANDILDYKDDDDKV Chromatograms: https://cdn.origene.com/chromatograms/mk6125_c07.zip Restriction Sites: SgfI-MluI This product is to be used for laboratory only. Not for diagnostic or therapeutic use. View online » ©2021 OriGene -

Urocortin 2 Modulates Glucose Utilization and Insulin Sensitivity in Skeletal Muscle
Urocortin 2 modulates glucose utilization and insulin sensitivity in skeletal muscle Alon Chen*†, Bhawanjit Brar*, Cheol Soo Choi‡, David Rousso*, Joan Vaughan*, Yael Kuperman†, Shee Ne Kim‡, Cindy Donaldson*, Sean M. Smith*, Pauline Jamieson*, Chien Li*, Tim R. Nagy§, Gerald I. Shulman‡, Kuo-Fen Lee*, and Wylie Vale*¶ *Clayton Foundation Laboratories for Peptide Biology, The Salk Institute for Biological Studies, La Jolla, CA 92037; †Department of Neurobiology, The Weizmann Institute of Science, Rehovot 76100, Israel; ‡Department of Internal Medicine and Cellular and Molecular Physiology and Howard Hughes Medical Institute, Yale University School of Medicine, New Haven, CT 06536; and §Department of Nutrition Sciences, University of Alabama at Birmingham, Birmingham, AL 35294 Contributed by Wylie Vale, August 28, 2006 Skeletal muscle is the principal tissue responsible for insulin- Results and Discussion stimulated glucose disposal and is a major site of peripheral insulin Generation of Ucn 2-Deficient Mice. To explore the physiological resistance. Urocortin 2 (Ucn 2), a member of the corticotropin- role of Ucn 2, we generated mice that are deficient in this releasing factor (CRF) family, and its cognate type 2 CRF receptor peptide. A genomic DNA clone containing Ucn 2 was isolated, (CRFR2) are highly expressed in skeletal muscle. To determine the and a targeting construct in which the full Ucn 2 coding sequence physiological role of Ucn 2, we generated mice that are deficient in was replaced with a neomycin-resistant gene cassette was gen- this peptide. Using glucose-tolerance tests (GTTs), insulin-tolerance erated (Fig. 1a). J1 ES cells were electroporated with the tests (ITTs), and hyperinsulinemic euglycemic glucose clamp stud- targeting construct and were selected as described in ref. -

UC Davis UC Davis Previously Published Works
UC Davis UC Davis Previously Published Works Title Cardiac CRFR1 Expression Is Elevated in Human Heart Failure and Modulated by Genetic Variation and Alternative Splicing. Permalink https://escholarship.org/uc/item/8r73t67n Journal Endocrinology, 157(12) ISSN 0013-7227 Authors Pilbrow, Anna P Lewis, Kathy A Perrin, Marilyn H et al. Publication Date 2016-12-01 DOI 10.1210/en.2016-1448 License https://creativecommons.org/licenses/by-nc-nd/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Manuscript (MUST INCLUDE TITLE PAGE AND ABSTRACT) Click here to download Manuscript (MUST INCLUDE TITLE PAGE AND ABSTRACT) Endocrinology CRFR1 ms.docx 1 Myocardial expression of Corticotropin-Releasing Factor Receptor 1 (CRFR1) is elevated in human 2 heart failure and is modulated by genetic variation and a novel CRFR1 splice variant. 3 4 Anna P Pilbrow1,2,* PhD, Kathy A Lewis1 BS, Marilyn H Perrin1 PhD, Wendy E Sweet3 MS, Christine S 5 Moravec3 PhD, WH Wilson Tang3 MD, Mark O Huising1 PhD, Richard W Troughton2 MD PhD and 6 Vicky A Cameron2 PhD. 7 8 1. Peptide Biology Laboratories, The Salk Institute for Biological Studies, 10010 North Torrey Pines 9 Road, La Jolla, CA 92037, USA. 10 2. Christchurch Heart Institute, Department of Medicine, University of Otago, Christchurch, 2 11 Riccarton Avenue, PO Box 4345, Christchurch 8011, New Zealand. 12 3. Kaufman Center for Heart Failure, Department of Cardiovascular Medicine, Cleveland Clinic, 9500 13 Euclid Avenue, Cleveland, OH 44195, USA. 14 15 Abbreviated title: CRFR1 in human heart failure 16 Keywords: heart failure, CRFR1, CRHR1, alternative splicing, splice variant, polymorphism, human. -

Mechanism of Astragalus Membranaceus in the Treatment Of
www.nature.com/scientificreports OPEN Mechanism of Astragalus membranaceus in the treatment of laryngeal cancer based on gene co‑expression network and molecular docking Kai Feng Dong1,5, Meng Qi Huo4,5, Heng Ya Sun2, Tian Ke Li3 & Dan Li1* Astragalus membranaceus (HUANG QI, HQ) is a kind of traditional Chinese medicine. Researchers have widely concerned its antitumor efect. At present, there is still a lack of research on the treatment of laryngeal cancer with HQ. In this study, we integrated data from the weighted gene co-expression network of laryngeal cancer samples and the components and targets of HQ. A new method for dividing PPI network modules is proposed. Important targets of HQ treatment for laryngeal cancer were obtained through the screening of critical modules. These nodes performed diferential expression analysis and survival analysis through external data sets. GSEA enrichment analysis reveals pathways for important targets participation. Finally, molecular docking screened active ingredients in HQ that could interact with important targets. Combined with the laryngeal cancer gene co expression network and HQ PPI network, we obtained the critical module related to laryngeal cancer. Among them, MMP1, MMP3, and MMP10 were chosen as important targets. External data sets demonstrate that their expression in tumor samples is signifcantly higher than in normal samples. The survival time of patients with high expression group was signifcantly shortened, which is a negative factor for prognosis. GSEA enrichment analysis found that they are mainly involved in tumor-related pathways such as ECM receptor interaction and Small cell lung cancer. The docking results show that the components that can well bind to important targets of HQ are quercetin, rutin, and Chlorogenic acid, which may be the primary mechanism of the anti-cancer efect of HQ. -

Anti-UTS2 Polyclonal Antibody (DPAB3155RH) This Product Is for Research Use Only and Is Not Intended for Diagnostic Use
Anti-UTS2 polyclonal antibody (DPAB3155RH) This product is for research use only and is not intended for diagnostic use. PRODUCT INFORMATION Product Overview Polyclonal antibody to human Urotensin II Antigen Description Urotensin-2 is a protein that in humans is encoded by the UTS2 gene. This genetic encodes an invidious mature peptide that is an active cyclic homogeneous heptapeptide absolutely conserved from lamprey to human. The active cyclic peptide acts as a vaso-constrictor and is expressed only in brain tissue. Despite the genetic family name similarity, this gene is not homologous to urocortine, a member of the sauvagine/corticotropin-releasing factor/urotensin I family. Most of the pro-protein is analogously cleaved to make the mature peptide. Transcript variants encoding different preproprotein endocrine isoforms have been described for this genetic structure. Specificity Synthetic human Urotensin II. There were no cross reactivities obtained with human Endothelin (aa 1-21) (< 0,1%); human big Endothelin (aa 1-38) (< 0,1%); human Urodilatin; human ANP (1- 28) (< 0,1%); human BNP (aa 1-32) (< 0,1%); Adrenomedulin Immunogen Synthetic human Urotensin II, KLH conjugated (ETPDCFWKYCV) Isotype IgG Source/Host Rabbit Species Reactivity Human Conjugate Unconjugated Applications ELISA Concentration 20 μl / 100 μl (lyophilized). Resuspend in 20 μl / 100 μl aqua bidest Preservative None Storage 2-8 oC (lyophilized); - 20 oC (dissolved). Repeated thawing and freezing must be avoided GENE INFORMATION Gene Name UTS2 urotensin 2 [Homo sapiens] -

Expression of Urocortin Peptides in Canine Myocardium and Plasma
Edinburgh Research Explorer Expression of urocortin peptides in canine myocardium and plasma Citation for published version: Veloso, GF, Ohad, DG, Francis, AJ, Vaughan, JM, Brownstein, DG, Culshaw, GJ, Vale, WW, French, AT & Jamieson, PM 2011, 'Expression of urocortin peptides in canine myocardium and plasma', The Veterinary Journal, vol. 188, no. 3, pp. 318-324. https://doi.org/10.1016/j.tvjl.2010.05.019 Digital Object Identifier (DOI): 10.1016/j.tvjl.2010.05.019 Link: Link to publication record in Edinburgh Research Explorer Document Version: Peer reviewed version Published In: The Veterinary Journal Publisher Rights Statement: © 2010 Elsevier Ltd General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 06. Oct. 2021 NIH Public Access Author Manuscript Vet J. Author manuscript; available in PMC 2012 June 1. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Vet J. 2011 June ; 188(3): 318±324. doi:10.1016/j.tvjl.2010.05.019. Expression of urocortin peptides in canine myocardium and plasma Gemma Fraga Velosoa, Dan G. -

Supplementary Materials
Supplementary materials Supplementary Table S1: MGNC compound library Ingredien Molecule Caco- Mol ID MW AlogP OB (%) BBB DL FASA- HL t Name Name 2 shengdi MOL012254 campesterol 400.8 7.63 37.58 1.34 0.98 0.7 0.21 20.2 shengdi MOL000519 coniferin 314.4 3.16 31.11 0.42 -0.2 0.3 0.27 74.6 beta- shengdi MOL000359 414.8 8.08 36.91 1.32 0.99 0.8 0.23 20.2 sitosterol pachymic shengdi MOL000289 528.9 6.54 33.63 0.1 -0.6 0.8 0 9.27 acid Poricoic acid shengdi MOL000291 484.7 5.64 30.52 -0.08 -0.9 0.8 0 8.67 B Chrysanthem shengdi MOL004492 585 8.24 38.72 0.51 -1 0.6 0.3 17.5 axanthin 20- shengdi MOL011455 Hexadecano 418.6 1.91 32.7 -0.24 -0.4 0.7 0.29 104 ylingenol huanglian MOL001454 berberine 336.4 3.45 36.86 1.24 0.57 0.8 0.19 6.57 huanglian MOL013352 Obacunone 454.6 2.68 43.29 0.01 -0.4 0.8 0.31 -13 huanglian MOL002894 berberrubine 322.4 3.2 35.74 1.07 0.17 0.7 0.24 6.46 huanglian MOL002897 epiberberine 336.4 3.45 43.09 1.17 0.4 0.8 0.19 6.1 huanglian MOL002903 (R)-Canadine 339.4 3.4 55.37 1.04 0.57 0.8 0.2 6.41 huanglian MOL002904 Berlambine 351.4 2.49 36.68 0.97 0.17 0.8 0.28 7.33 Corchorosid huanglian MOL002907 404.6 1.34 105 -0.91 -1.3 0.8 0.29 6.68 e A_qt Magnogrand huanglian MOL000622 266.4 1.18 63.71 0.02 -0.2 0.2 0.3 3.17 iolide huanglian MOL000762 Palmidin A 510.5 4.52 35.36 -0.38 -1.5 0.7 0.39 33.2 huanglian MOL000785 palmatine 352.4 3.65 64.6 1.33 0.37 0.7 0.13 2.25 huanglian MOL000098 quercetin 302.3 1.5 46.43 0.05 -0.8 0.3 0.38 14.4 huanglian MOL001458 coptisine 320.3 3.25 30.67 1.21 0.32 0.9 0.26 9.33 huanglian MOL002668 Worenine