1 ARTICLE TYPE 1 Research Paper 2 3 TITLE 4 Interpreting Vegetation Change in Tropical Arid Ecosystems from Sediment Molecular F
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ENVIRONMENTAL SCOPING DOCUMENT Pilbara Iron Ore & Infrastructure Project
ENVIRONMENTAL SCOPING DOCUMENT Pilbara Iron Ore & Infrastructure Project: East-West Railway and Mine Sites (Stage B) (Assessment No. 1520) for Fortescue Metals Group Limited ENVIRON AUSTRALIA PTY LTD FORTESCUE METALS GROUP LIMITED Suite 7, The Russell Centre 159 Adelaide Terrace Fortescue House East Perth WA 6004 50 Kings Park Road, West Perth Tel.: +618 9225 5199 Western Australia 6005 Fax: +618 9225 5155 Tel.: +618 9266 0111 A.C.N. 095 437 442 Fax: +618 9266 0188 A.C.N. 57 002 594 872 Ref: Stage B Scoping final 16 Nov 04 16 November 2004 Environmental Scoping Document Pilbara Iron Ore & Infrastructure Project: East-West railway and Mine Sites (Stage B) 16 November 2004 for Fortescue Metals Group Limited Page i TABLE OF CONTENTS Page No. 1. INTRODUCTION....................................................................................................................1 1.1 THE PROPOSAL ....................................................................................................... 1 1.2 NEED FOR THE PROJECT..................................................................................... 3 1.3 PROJECT BENEFITS ............................................................................................... 3 1.4 PROPONENT ............................................................................................................. 4 2. PROJECT DESCRIPTION .................................................................................................... 5 2.1 PROJECT LOCATION ............................................................................................ -

Handbook of Western Australian Aboriginal Languages South of the Kimberley Region
PACIFIC LINGUISTICS Series C - 124 HANDBOOK OF WESTERN AUSTRALIAN ABORIGINAL LANGUAGES SOUTH OF THE KIMBERLEY REGION Nicholas Thieberger Department of Linguistics Research School of Pacific Studies THE AUSTRALIAN NATIONAL UNIVERSITY Thieberger, N. Handbook of Western Australian Aboriginal languages south of the Kimberley Region. C-124, viii + 416 pages. Pacific Linguistics, The Australian National University, 1993. DOI:10.15144/PL-C124.cover ©1993 Pacific Linguistics and/or the author(s). Online edition licensed 2015 CC BY-SA 4.0, with permission of PL. A sealang.net/CRCL initiative. Pacific Linguistics is issued through the Linguistic Circle of Canberra and consists of four series: SERIES A: Occasional Papers SERIES c: Books SERIES B: Monographs SERIES D: Special Publications FOUNDING EDITOR: S.A. Wurm EDITORIAL BOARD: T.E. Dutton, A.K. Pawley, M.D. Ross, D.T. Tryon EDITORIAL ADVISERS: B.W.Bender KA. McElhanon University of Hawaii Summer Institute of Linguistics DavidBradley H.P. McKaughan La Trobe University University of Hawaii Michael G. Clyne P. Miihlhausler Monash University University of Adelaide S.H. Elbert G.N. O'Grady University of Hawaii University of Victoria, B.C. KJ. Franklin KL. Pike Summer Institute of Linguistics Summer Institute of Linguistics W.W.Glover E.C. Polome Summer Institute of Linguistics University of Texas G.W.Grace Gillian Sankoff University of Hawaii University of Pennsylvania M.A.K Halliday W.A.L. Stokhof University of Sydney University of Leiden E. Haugen B.K T' sou Harvard University City Polytechnic of Hong Kong A. Healey E.M. Uhlenbeck Summer Institute of Linguistics University of Leiden L.A. -
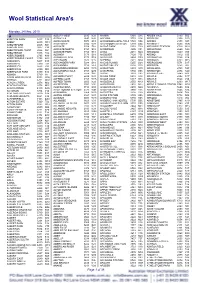
Wool Statistical Area's
Wool Statistical Area's Monday, 24 May, 2010 A ALBURY WEST 2640 N28 ANAMA 5464 S15 ARDEN VALE 5433 S05 ABBETON PARK 5417 S15 ALDAVILLA 2440 N42 ANCONA 3715 V14 ARDGLEN 2338 N20 ABBEY 6280 W18 ALDERSGATE 5070 S18 ANDAMOOKA OPALFIELDS5722 S04 ARDING 2358 N03 ABBOTSFORD 2046 N21 ALDERSYDE 6306 W11 ANDAMOOKA STATION 5720 S04 ARDINGLY 6630 W06 ABBOTSFORD 3067 V30 ALDGATE 5154 S18 ANDAS PARK 5353 S19 ARDJORIE STATION 6728 W01 ABBOTSFORD POINT 2046 N21 ALDGATE NORTH 5154 S18 ANDERSON 3995 V31 ARDLETHAN 2665 N29 ABBOTSHAM 7315 T02 ALDGATE PARK 5154 S18 ANDO 2631 N24 ARDMONA 3629 V09 ABERCROMBIE 2795 N19 ALDINGA 5173 S18 ANDOVER 7120 T05 ARDNO 3312 V20 ABERCROMBIE CAVES 2795 N19 ALDINGA BEACH 5173 S18 ANDREWS 5454 S09 ARDONACHIE 3286 V24 ABERDEEN 5417 S15 ALECTOWN 2870 N15 ANEMBO 2621 N24 ARDROSS 6153 W15 ABERDEEN 7310 T02 ALEXANDER PARK 5039 S18 ANGAS PLAINS 5255 S20 ARDROSSAN 5571 S17 ABERFELDY 3825 V33 ALEXANDRA 3714 V14 ANGAS VALLEY 5238 S25 AREEGRA 3480 V02 ABERFOYLE 2350 N03 ALEXANDRA BRIDGE 6288 W18 ANGASTON 5353 S19 ARGALONG 2720 N27 ABERFOYLE PARK 5159 S18 ALEXANDRA HILLS 4161 Q30 ANGEPENA 5732 S05 ARGENTON 2284 N20 ABINGA 5710 18 ALFORD 5554 S16 ANGIP 3393 V02 ARGENTS HILL 2449 N01 ABROLHOS ISLANDS 6532 W06 ALFORDS POINT 2234 N21 ANGLE PARK 5010 S18 ARGYLE 2852 N17 ABYDOS 6721 W02 ALFRED COVE 6154 W15 ANGLE VALE 5117 S18 ARGYLE 3523 V15 ACACIA CREEK 2476 N02 ALFRED TOWN 2650 N29 ANGLEDALE 2550 N43 ARGYLE 6239 W17 ACACIA PLATEAU 2476 N02 ALFREDTON 3350 V26 ANGLEDOOL 2832 N12 ARGYLE DOWNS STATION6743 W01 ACACIA RIDGE 4110 Q30 ALGEBUCKINA -

An Assessment of the Impact of Ophthalmia Dam on the Floodplains of the Fortescue River on Ethel Creek and Roy Hill Stations
Research Library Resource management technical reports Natural resources research 1-8-1999 An assessment of the impact of Ophthalmia Dam on the floodplains of the orF tescue River on Ethel Creek and Roy Hill Stations A L. Payne A A. Mitchell Follow this and additional works at: https://researchlibrary.agric.wa.gov.au/rmtr Part of the Environmental Indicators and Impact Assessment Commons Recommended Citation Payne, A L, and Mitchell, A A. (1999), An assessment of the impact of Ophthalmia Dam on the floodplains of the Fortescue River on Ethel Creek and Roy Hill Stations. Department of Primary Industries and Regional Development, Western Australia, Perth. Report 124. This report is brought to you for free and open access by the Natural resources research at Research Library. It has been accepted for inclusion in Resource management technical reports by an authorized administrator of Research Library. For more information, please contact [email protected]. ISSN 0729-3135 August 1999 An Assessment of the Impact of Ophthalmia Dam on the Floodplains of the Fortescue River on Ethel Creek and Roy Hill Stations A.L. Payne and A.A. Mitchell Resource Management Technical Report No.124 IMPACT OF OPHTHALMIA DAM ON THE FLOODPLAINS OF THE FORTESCUE RIVER Disclaimer The contents of this report were based on the best available information at the time of publication. It is based in part on various assumptions and predictions. Conditions may change over time and conclusions should be interpreted in the light of the latest information available. © Director General, Department of Agriculture Western Australia 2004 ii IMPACT OF OPHTHALMIA DAM ON THE FLOODPLAINS OF THE FORTESCUE RIVER Information for Contributors Scientists who wish to publish the results of their investigations have access to a large number of journals. -

OF No, 33) PERTH
E16333 OF (Published by Authority at 3 .30 p.m.) No, 33) PERTH : FRIDAY, 3rd JUNE [1977 Liquor Act Amendment Act, 1976 . Superannuation and Family Benefits Act, PROCLAMATION Amendment Act, 1976. WESTERN AUSTRALIA, jBy His Excellency Air Chief Marshal Sir Wallace To Wit: jKyle, Knight Grand Cross of the Most Honourable PROCLAMATION WALLACE KYLE, Order of the Bath, Knight Commander of the Governor. Royal Victorian Order, Commander of the Most WESTERN AUSTRALIA, )By His Excellency Air Chief Marshal Sir Wallace [L.S .] Excellent Order of the British Empire, Companion To Wit: Kyle, Knight Grand Cross of the Most Honourable of the Distinguished Service Order, Distinguished WALLACE KYLE, Order of the Bath, Knight Commander of the Flying Cross, Knight of Grace of the Most Governor . Royal Victorian Order, Commander of the Most Venerable Order of the Hospital of St . John of CL.S .7 Excellent Order of the British Empire, Companion Jerusalem, Governor in and over the State of of the Distinguished Service Order, Distinguished Western Australia and its dependencies in the Flying Cross, Knight of Grace of the Most Commonwealth of Australia. Venerable Order of the Hospital of St . John of Jerusalem, Governor in and over the State of WHEREAS it is enacted by subsection (3) of Western Australia and its dependencies in the section 2 of the Liquor Act Amendment Act, 1976, Commonwealth of Australia. that the provisions of that Act, other than sections 5 and 33, shall come into operation on such date WHEREAS it is enacted by subsection (2) of or dates as -
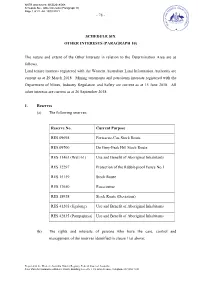
Schedule Six Other Interests (Paragraph 10)
NNTR attachment: WCD2018/008 Schedule Six - Other Interests (Paragraph 10) Page 1 of 22, A4, 19/01/2021 - 78 - SCHEDULE SIX OTHER INTERESTS (PARAGRAPH 10) The nature and extent of the Other Interests in relation to the Determination Area are as follows. Land tenure interests registered with the Western Australian Land Information Authority are current as at 29 March 2018. Mining tenements and petroleum interests registered with the Department of Mines, Industry Regulation and Safety are current as at 15 June 2018. All other interests are current as at 26 September 2018. 1. Reserves (a) The following reserves: Reserve No. Current Purpose RES 09698 Fortescue-Cue Stock Route RES 09700 De Grey-Peak Hill Stock Route RES 11463 (Well 61) Use and Benefit of Aboriginal Inhabitants RES 12297 Protection of the Rabbit-proof Fence No.1 RES 15159 Stock Route RES 17650 Racecourse RES 18938 Stock Route (Deviation) RES 41265 (Jigalong) Use and Benefit of Aboriginal Inhabitants RES 42835 (Parnpajinya) Use and Benefit of Aboriginal Inhabitants (b) The rights and interests of persons who have the care, control and management of the reserves identified in clause 1(a) above; Prepared in the Western Australia District Registry, Federal Court of Australia Peter Durack Commonwealth Law Courts Building, Level 6, 1 Victoria Avenue, Telephone 08 9268 7100 NNTR attachment: WCD2018/008 Schedule Six - Other Interests (Paragraph 10) Page 2 of 22, A4, 19/01/2021 - 79 - (c) The rights and interests of persons entitled to access and use the reserves identified in clause 1(a) above for the respective purposes for which they are reserved, subject to any statutory limitations upon those rights; and (d) The rights and interests of persons holding leases over areas of the reserves identified in clause 1(a) above, if any. -

1 Title: 1 Evidence for Extreme Floods in Arid Subtropical Northwest Australia During the Little Ice Age 2 Chronozone (CE 1400
*Manuscript Click here to view linked References 1 Title: 2 Evidence for extreme floods in arid subtropical northwest Australia during the Little Ice Age 3 Chronozone (CE 1400–1850) 4 5 Authors 6 A. Rouillard*a1, G. Skrzypeka, C. Turneyb, S. Dogramacia,c, Q. Huad, A. Zawadzkid, J. Reevese, P. 7 Greenwooda,f,g, A.J. O’Donnella and P.F. Griersona 8 9 aEcosystems Research Group and West Australian Biogeochemistry Centre, School of Plant 10 Biology, The University of Western Australia, Crawley, WA 6009, Australia 11 bClimate Change Research Centre, School of Biological, Earth and Environmental Sciences, 12 University of New South Wales, Sydney, NSW 2052, Australia 13 cRio Tinto Iron Ore, Perth, WA 6000, Australia 14 dAustralian Nuclear Science and Technology Organisation (ANSTO), Locked Bag 2001, Kirrawee 15 DC, NSW 2232, Australia 16 eFaculty of Science and Technology, Federation University Australia, PO Box 663, Ballarat VIC 17 3353, Australia 18 fCentre for Exploration Targeting, School of Earth and Environment, The University of Western 19 Australia, Crawley, WA, Australia 20 gWestern Australia Organic and Isotope Geochemistry Centre, The Institute for Geoscience 21 Research, Department of Chemistry, Curtin University, Bentley, WA, Australia 22 23 *author for correspondence: [email protected]; 24 [email protected] 1 Present email and address: [email protected]; Centre for GeoGenetics, Natural History Museum of Denmark, University of Copenhagen, Øster Voldgade 5-7, 1350 Copenhagen K, Denmark Abbreviations: Common Era (CE); Constant Initial Concentration (CIC); Constant Rate of Supply (CRS); El Niño-Southern 1 Oscillation (ENSO); Indonesian Australian Summer Monsoon (IASM); Inorganic Carbon (IC); Intertropical Convergence Zone (ITCZ); Little Ice Age (LIA); Madden-Julian/Intra-seasonal Oscillation (MJO); Northern Hemisphere (NH); Organic Carbon (OC); Organic Matter (OM); Southern Annular Mode (SAM); Southern Hemisphere (SH); Tropical Cyclone (TC). -

Official/Settler Names Index
Official/Settler Location Station/Mission/Reserve File Number Abraham, Mr 313/1904 Abraham, Mr J.S. West Perth 421/1906 Abraham, Percy 557/1903 Abraham, Percy Nor West 421/1906 Adam, J.P. York 319/1901 Adam, John (RM Northam) Northam 588/1899 Adam, John (RM Northam) Northam 471/1898 Adam, Mr 387/1898 Adam, W.H. (RM Katanning) Katanning 15/1898 Adam, W.K. (RM Katanning) Katanning 185/1899 Adam, W.K. (RM Katanning) Katanning 441/1898 Adam, W.K. (RM Katanning) Katanning 342/1900 Adam, W.K. (RM Katanning) Katanning 330/1898 Adam, W.K. (RM Katanning) Katanning 150/1899 Adam, W.K. (RM Kattanning) Katanning 353/1898 Adams (Const. No. 202) Dongarra 666/1906 Adams, Arthur R. (Actg. RM Onslow) Onslow 612/1907 Adams, Arthur R. (RM Derby) Derby Unnumbered/1908 Adams, Arthur R. (RM Derby) Derby 957/1908 Adams, Dr (DMO) Derby 799/1908 Adams, Jane Mangowine 279/1900 Adams, Jane 665/1898 Adams, Jane Mangonine 93/1905 Adams, Mr Derby 797/1908 Adams, Mr Onslow 349/1908 Adams, Mr (RM Derby) Derby 409B/1908 Adams, Mr J. Mangowine 279/1901 Adams, Mrs Yanajin Station Yanajin Station 1098/1906 Adams, Mrs Shark Bay 11/1905 Adams, W.J. Dongarra 328/1908 Adams, W.J. (Const. No. 202) Dongarra Unnumbered/1908 Adams, W.J. (Const. No. 202) 442/1901 Adcock, Mr C.J. Derby 616/1902 Adcock, Mrs Derby 762/1907 Adcock, Mrs (nee Thompson) Derby 616/1902 Ah Chew (m-Malay) Quanborn Station (?) Quanborn Station (?) 835/1908 Ahern, H.N. Twenty Mile Sandy 647/1902 Aikman, Andy Cook Creek 284/1908 Aitchison, J. -

Nuytsia WESTERN AUSTRALIA's JOURNAL of SYSTEMATIC BOTANY ISSN 0085–4417
Nuytsia WESTERN AUSTRALIA'S JOURNAL OF SYSTEMATIC BOTANY ISSN 0085–4417 Maslin, B.R. & van Leeuwen, S. New taxa of Acacia (Leguminosae: Mimosoideae) and notes on other species from the Pilbara and adjacent desert regions of Western Australia Nuytsia 18: 139–188 (2008) All enquiries and manuscripts should be directed to: The Managing Editor – NUYTSIA Western Australian Herbarium Telephone: +61 8 9334 0500 Dept of Environment and Conservation Facsimile: +61 8 9334 0515 Locked Bag 104 Bentley Delivery Centre Email: [email protected] Western Australia 6983 Web: science.dec.wa.gov.au/nuytsia AUSTRALIA All material in this journal is copyright and may not be reproduced except with the written permission of the publishers. © Copyright Department of Environment and Conservation B.R.Nuytsia Maslin 18: 139–188 & S. van (2008) Leeuwen, New taxa of Acacia from the Pilbara 139 New taxa of Acacia (Leguminosae: Mimosoideae) and notes on other species from the Pilbara and adjacent desert regions of WesternAustralia Bruce R. Maslin1 and Stephen van Leeuwen2 1Western Australian Herbarium, Department of Environment and Conservation, Locked Bag 104, Bentley Delivery Centre, Western Australia 6983 2Woodvale Research Centre, Science Division, Departrment of Environment and Conservation, PO Box 51, Wanneroo, Western Australia 6946 Abstract Maslin, B.R. & van Leeuwen, S. New taxa of Acacia (Leguminosae: Mimosoideae) and notes on other species from the Pilbara and adjacent desert regions of Western Australia. Nuytsia 18: 139–188 (2008). Preparatory to publishing books on the Acacia Mill. flora of the Pilbara region, 10 new species (A. bromilowiana Maslin, A. fecunda Maslin, A. leeuweniana Maslin, A. -

Extreme Flood Regime of Northwest Australia
Discussion Paper | Discussion Paper | Discussion Paper | Discussion Paper | Hydrol. Earth Syst. Sci. Discuss., 11, 11905–11943, 2014 www.hydrol-earth-syst-sci-discuss.net/11/11905/2014/ doi:10.5194/hessd-11-11905-2014 HESSD © Author(s) 2014. CC Attribution 3.0 License. 11, 11905–11943, 2014 This discussion paper is/has been under review for the journal Hydrology and Earth System Extreme flood regime Sciences (HESS). Please refer to the corresponding final paper in HESS if available. of northwest Australia Impacts of a changing climate on a A. Rouillard et al. century of extreme flood regime of northwest Australia Title Page Abstract Introduction 1 1 1,2 3 1 A. Rouillard , G. Skrzypek , S. Dogramaci , C. Turney , and P. F. Grierson Conclusions References 1 West Australian Biogeochemistry Centre and Ecosystems Research Group, School of Plant Tables Figures Biology, The University of Western Australia, Crawley, WA 6009, Australia 2Rio Tinto Iron Ore, Perth, WA 6000, Australia 3Climate Change Research Centre, University of NSW, Sydney, NSW 2052, Australia J I J I Received: 7 October 2014 – Accepted: 9 October 2014 – Published: 24 October 2014 Correspondence to: A. Rouillard ([email protected], Back Close [email protected]) Full Screen / Esc Published by Copernicus Publications on behalf of the European Geosciences Union. Printer-friendly Version Interactive Discussion 11905 Discussion Paper | Discussion Paper | Discussion Paper | Discussion Paper | Abstract HESSD Globally, there has been much recent effort to improve understanding of climate change-related shifts in rainfall patterns, variability and extremes. Comparatively little 11, 11905–11943, 2014 work have focused on how such shifts might be altering hydrological regimes within 5 arid regional basins, where impacts are expected to be most significant. -

Aborigines Department
1901. WESTERN AUSTRALIA. ABORIGINES DEPARTMENT. REPORTFOE FINANCIAL YEAR ENDING 30TH JUNE, 1901. Presented to both Houses of Parliament by His Excellency's Command. PERTH: BY AUTHORITY WM. ALFRED WATSON, GOVERNMENT PRINTER. 1901. No. 26. Digitised by AIATSIS Library 2008-www.aiatsis.gov.au/library ABORIGINES DEPARTMENT. Report for Financial Year ending 30th June, 1901. To THE HON. THE PBEMIEB. SIB, I beg to submit my Report on the working of the Aborigines Department for the year ending 80th June, 1901. The general condition of the aborigines throughout the State has not been much altered since my last Report, but a great deal more information as to the details of their employment has bt'en rained from the reports of the Travelling Inspector, who has now completed his tour of investigation in the Northern half of the State, and has already commenced his tour of the Southern half by starting again from Geraldton and going Eastward, through Talgoo and Mt. Magnet, towards Lawlers and Lake Way, from whence he will work down in a very zig-zag line towards Israelite Bay, and then Westwards to Perth. This trip will probably occupy him twelve months, or even more, and by that time we shall have a reliable official account of almost every station in the State on which natives are employed, or even congregate, which will not be required again for some time, and will be a most useful basis on which the general distribution of relief, etc., can be granted in detail. His reports will be found in the Appendix, together with a plan showing his route and the places at which he stopped. -

Ecohydrological Conceptualisation of the Marillana Creek Region
September 2015 Ecohydrological Conceptualisation of the Marillana Creek Region Submitted to: BHP Billiton Iron Ore Level 29, 125 St Georges Terrace PERTH WA 6000 This report has been prepared solely for the purpose of informing environmental impact assessment pursuant to the Environmental Protection Act 1986 (WA) and Environmental Protection and Biodiversity Conservation Act 1999 (Cth) and is not intended for use for any other purpose. No representation or warranty is given that project development associated with any or all of the disturbance indicated in this report will actually proceed. As project development is dependent upon future events, the outcome of which is uncertain and cannot be assured, actual development may vary materially from this report. Report Number. 137646040-002-R-Rev4 Distribution: REPORT 1 Electronic Copy - BHP Billiton Iron Ore 1 Electronic Copy - Golder Associates Pty Ltd ECOHYDROLOGY OF THE MARILLANA CREEK REGION EXECUTIVE SUMMARY An ecohydrological conceptualisation has been developed of the Marillana Creek Region, as part of a broader Strategic Environmental Assessment associated with BHP Billiton Iron Ore’s Pilbara Expansion. The overall objective was to provide an enhanced understanding of the ecohydrology, including the identification of hydrological processes that support the environment. The specific aims of the conceptualisation were to: Present the major hydrological (surface water and groundwater) elements and processes with a clear link to supporting data. Define the catchment area based on robust hydrological (surface water and groundwater) evidence which will inform boundaries of subsequent hydrological models. Present the ecological values and characteristics, in terms of vegetation associations, fauna habitats, threatened species and communities, waterholes, landforms and soils.