Idorsia Financial Report 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

From the Academy
FROM THE ACADEMY Joint American Academy of DermatologyeNational Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy Craig A. Elmets, MD (Co-Chair),a HenryW.Lim,MD,b Benjamin Stoff, MD, MA,c Cody Connor, MD,a Kelly M. Cordoro, MD,d Mark Lebwohl, MD,e AprilW.Armstrong,MD,MPH,f Dawn M. R. Davis, MD,g Boni E. Elewski, MD,a Joel M. Gelfand, MD, MSCE,h Kenneth B. Gordon, MD,i AliceB.Gottlieb,MD,PhD,j Daniel H. Kaplan, MD, PhD,k Arthur Kavanaugh, MD,l Matthew Kiselica, BA/BS,m Dario Kivelevitch, MD,n Neil J. Korman, MD, PhD,o Daniela Kroshinsky, MD, MPH,p Craig L. Leonardi, MD,q Jason Lichten, MD,m NehalN.Mehta,MD,MSCE,r Amy S. Paller, MD,s Sylvia L. Parra, MD,t Arun L. Pathy, MD,u Elizabeth A. Farley Prater, MD,v Reena N. Rupani, MD,e Michael Siegel, PhD,m BruceE.Strober,MD,PhD,w,x Emily B. Wong, MD,y Jashin J. Wu, MD,z Vidhya Hariharan, PhD,aa and Alan Menter, MD (Co-Chair)n Birmingham, Alabama; Detroit, Michigan; Atlanta, Georgia; San Francisco, Los Angeles, San Diego, and Irvine, California; New York, New York; Rochester, Minnesota; Philadelphia and Pittsburgh, Pennsylva- nia; Milwaukee, Wisconsin; Portland, Oregon; Dallas and San Antonio, Texas; Cleveland, Ohio; Boston, Massachusetts; St. Louis, Missouri; Bethesda, Maryland; Chicago and Rosemont, Illinois; Sumter, South Carolina; Centennial, Colorado; Oklahoma City, Oklahoma; Farmington, Connecticut; and Waterloo, Ontario, Canada Psoriasis is a chronic inflammatory disease involving multiple organ systems and affecting approximately 3.2% of the world’s population. -

Annual Report on Annual Reports 2016
ANNUAL REPORT ON ANNUAL REPORTS 2016 TOP 400 ANNUAL REPORTS WHO RANKS WHERE? 100 ANNUALS IN BRIEF BEST REPORTING PRACTICES Company Value > Report Value Annual Report on Annual Reports 2016 Contents Report rating scale 3 Top 400 annual reports 4 Who ranks where? 25 From ABB to ZTE 100 annuals in brief 58 From Abbott to Yamaha How important is the annual report today? 92 Views from Cecilia Ketels, Kellie Friery, Renee Carter, David Robinson, Kaevan Gazdar, Elena Moskvina, Thomas Rosenmayr, Rob Stangroom, Andrey Kozhevnikov, Ana Santamarina, Katie Holcomb, Ananda Jagoda Best practices on key report attibutes 100 Strategy, message, investor information, risks, style, online… How we make it 121 How is your report doing? The report scan 127 The report rating panel 128 Robert Berick, Susan Blesener, Renee Carter, Vero Escarmelle, Helena Fournial, Kaevan Gazdar, Mike Guillaume, Pradip Seth, Eva Wolosiuk Making reports pay off 133 e.com – ReportWatch 135 2 Report rating scale A+ ééééé First-rate A éééé(é) Excellent A- éééé Very good B+ ééé(é) Sound B ééé Average B- éé(é) Uneven C+ éé Common C é(é) Substandard C- é Poor D (é) Uncompetitive 3 Top 400 annual reports AkzoNobel (No. 1) Electrolux (No. 2 ) SCA (No. 3) Volvo (No. 4) 4 Report rank Company Country Report rating Compare 1 AKZONOBEL Netherlands A+ DUPONT 2 ELECTROLUX Sweden A+ WHIRLPOOL 3 SCA Sweden A+ KIMBERLY-CLARK 4 VOLVO Sweden A+ DAIMLER 5 POTASHCORP Canada A+ AGRIUM 6 ATLAS COPCO Sweden A SANDVIK 7 STORA ENSO Finland A UPM 8 BOLIDEN Sweden A GLENCORE 9 WIENERBERGER Austria A BORAL 10 -
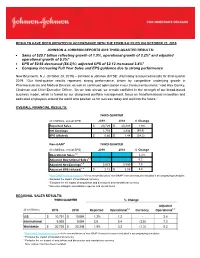
Sales of $20.7 Billion Reflecting Growth of 1.9%, Operational Growth of 3.2
RESULTS HAVE BEEN UPDATED IN ACCORDANCE WITH THE FORM 8-K FILED ON OCTOBER 23, 2019 JOHNSON & JOHNSON REPORTS 2019 THIRD-QUARTER RESULTS: • Sales of $20.7 billion reflecting growth of 1.9%, operational growth of 3.2%* and adjusted operational growth of 5.2%* • EPS of $0.66 decreased (54.2)%; adjusted EPS of $2.12 increased 3.4%* • Company increasing Full Year Sales and EPS guidance due to strong performance New Brunswick, N.J. (October 23, 2019) – Johnson & Johnson (NYSE: JNJ) today announced results for third-quarter 2019. “Our third-quarter results represent strong performance, driven by competitive underlying growth in Pharmaceuticals and Medical Devices, as well as continued optimization in our Consumer business,” said Alex Gorsky, Chairman and Chief Executive Officer. “As we look ahead, we remain confident in the strength of our broad-based business model, which is fueled by our disciplined portfolio management, focus on transformational innovation and dedicated employees around the world who position us for success today and well into the future.” OVERALL FINANCIAL RESULTS: THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Reported Sales $ 20,729 $ 20,348 1.9% Net Earnings 1,753 3,934 (55.4) EPS (diluted) $ 0.66 $ 1.44 (54.2) Non-GAAP* THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Operational Sales1,2 3.2% Adjusted Operational Sales1,3 5.2 Adjusted Net Earnings1,4 5,672 5,590 1.5 Adjusted EPS (diluted)1,4 $ 2.12 $ 2.05 3.4 1 Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures -

FDA Listing of Authorized Generics As of July 1, 2021
FDA Listing of Authorized Generics as of July 1, 2021 Note: This list of authorized generic drugs (AGs) was created from a manual review of FDA’s database of annual reports submitted to the FDA since January 1, 1999 by sponsors of new drug applications (NDAs). Because the annual reports seldom indicate the date an AG initially entered the market, the column headed “Date Authorized Generic Entered Market” reflects the period covered by the annual report in which the AG was first reported. Subsequent marketing dates by the same firm or other firms are not included in this listing. As noted, in many cases FDA does not have information on whether the AG is still marketed and, if not still marketed, the date marketing ceased. Although attempts have been made to ensure that this list is as accurate as possible, given the volume of d ata reviewed and the possibility of database limitations or errors, users of this list are cautioned to independently verify the information on the list. We welcome comments on and suggested changes (e.g., additions and deletions) to the list, but the list may include only information that is included in an annual report. Please send suggested changes to the list, along with any supporting documentation to: [email protected] A B C D E F G H I J K L M N O P Q R S T U V X Y Z NDA Applicant Date Authorized Generic Proprietary Name Dosage Form Strength Name Entered the Market 1 ACANYA Gel 1.2% / 2.5% Bausch Health 07/2018 Americas, Inc. -

Annual Report
ANNUAL REPORT 2019 MARCH 2020 To Our Shareholders Alex Gorsky Chairman and Chief Executive Officer By just about every measure, Johnson & These are some of the many financial and Johnson’s 133rd year was extraordinary. strategic achievements that were made possible by the commitment of our more than • We delivered strong operational revenue and 132,000 Johnson & Johnson colleagues, who adjusted operational earnings growth* that passionately lead the way in improving the health exceeded the financial performance goals we and well-being of people around the world. set for the Company at the start of 2019. • We again made record investments in research and development (R&D)—more than $11 billion across our Pharmaceutical, Medical Devices Propelled by our people, products, and and Consumer businesses—as we maintained a purpose, we look forward to the future relentless pursuit of innovation to develop vital with great confidence and optimism scientific breakthroughs. as we remain committed to leading • We proudly launched new transformational across the spectrum of healthcare. medicines for untreated and treatment-resistant diseases, while gaining approvals for new uses of many of our medicines already in the market. Through proactive leadership across our enterprise, we navigated a constant surge • We deployed approximately $7 billion, of unique and complex challenges, spanning primarily in transactions that fortify our dynamic global issues, shifting political commitment to digital surgery for a more climates, industry and competitive headwinds, personalized and elevated standard of and an ongoing litigious environment. healthcare, and that enhance our position in consumer skin health. As we have experienced for 133 years, we • And our teams around the world continued can be sure that 2020 will present a new set of working to address pressing public health opportunities and challenges. -

Johnson & Johnson Annonce Que L'exécution De L'offre Publique D
Press Contacts: Amy Jo Meyer Johnson & Johnson annonce que l'Exécution de l'Offre Publique +1 (732) 524 6678 d'Acquisition visant Actelion est prévue pour le 16 juin 2017 [email protected] Annonce avoir reçu toutes les autorisations réglementaires nécessaires à Ernie Knewitz +1 (917) 697-2318 l'exécution de l'acquisition d'Actelion [email protected] Investor Contacts: NEW BRUNSWICK, N.J. – 9 juin 2017 – Johnson & Johnson (NYSE:JNJ) a Joseph J. Wolk annoncé aujourd'hui qu'avec la réception, ce jour, de l'approbation de l'acquisition +1 (732) 524-1142 projetée visant Actelion Ltd (SIX : ATLN) par la Commission européenne (CE), toutes les autorisations réglementaires nécessaires à l'exécution de la transaction Lesley Fishman +1 (732) 524-3922 ont été reçues. Johnson & Johnson s'attend à ce que l'exécution de l'offre publique d'acquisition entièrement en espèces de sa filiale suisse Janssen Holding GmbH, portant sur toutes les actions en mains du public d'Actelion Ltd au prix de $280 par action, payable en dollars américains, ait lieu le 16 juin 2017. Comme annoncé précédemment, dans le cadre de la transaction, Actelion transférera ses activités en matière de recherche médicamenteuse et ses actifs relatifs au développement préclinique dans une société biopharmaceutique suisse nouvellement constituée ("Idorsia Ltd"). Il est prévu que les actions d'Idorsia Ltd soient distribuées aux actionnaires d'Actelion au moyen d'un dividende en nature et qu'elles soient cotées à la SIX Swiss Exchange le jour de l'exécution de l'offre publique d'acquisition. Une filiale de Johnson & Johnson détiendra initialement 9.9 pour cent des actions d'Idorsia Ltd et elle dispose du droit d'éventuellement augmenter sa participation jusqu'à concurrence de 32 pour cent au moyen d'une obligation convertible. -

HIGHLIGHTS of PRESCRIBING INFORMATION TRACLEER® (Bosentan) Tablets These Highlights Do Not Include All the Information Needed to Use TRACLEER Safely and Effectively
HIGHLIGHTS OF PRESCRIBING INFORMATION TRACLEER® (bosentan) tablets These highlights do not include all the information needed to use TRACLEER safely and effectively. See full prescribing information for TRACLEER. • Reduce the dose and closely monitor patients developing aminotransferase TRACLEER® (bosentan) tablets, for oral use elevations more than 3 X Upper Limit of Normal (ULN) (2.1). ® TRACLEER (bosentan) tablets for oral suspension --------------------------- DOSAGE FORMS AND STRENGTHS --------------------------- Initial U.S. Approval: 2001 • Film-coated tablet: 62.5 mg and 125 mg (3) WARNING: RISKS OF HEPATOTOXICITY and EMBRYO-FETAL TOXICITY • Tablet for oral suspension: 32 mg (3) See full prescribing information for complete boxed warning. ----------------------------------- CONTRAINDICATIONS ----------------------------------- TRACLEER is available only through a restricted distribution program called • Pregnancy (4.1) the Bosentan REMS Program because of these risks (5.3): • Use with Cyclosporine A (4.2) Elevations of liver aminotransferases (ALT, AST) and liver failure have been • Use with Glyburide (4.3) reported with TRACLEER (5.1). • Hypersensitivity (4.4) • Measure liver aminotransferases prior to initiation of treatment and then -----------------------------WARNINGS AND PRECAUTIONS ----------------------------- monthly (2.1, 5.1). • Fluid retention: May require intervention (5.4). • Discontinue TRACLEER if aminotransferase elevations are accompanied by • Pulmonary veno-occlusive disease (PVOD): If signs of pulmonary edema signs or symptoms of liver dysfunction or injury or increases in bilirubin occur, consider the diagnosis of associated PVOD and consider discontinuing ≥2 x ULN (2.4, 5.1). TRACLEER (5.5). Based on animal data, TRACLEER is likely to cause major birth defects if used • Decreased sperm counts (5.6). during pregnancy (4.1, 5.2, 8.1). • Decreases in hemoglobin and hematocrit: Monitor hemoglobin levels after • Must exclude pregnancy before and during treatment (2.1, 4.1, 8.1). -

2016 Annual Report
ANNUAL REPORT 2016 MARCH 2017 TO OUR SHAREHOLDERS ALEX GORSKY Chairman and Chief Executive Officer I’ve worked in the health care industry for Rather, true innovations are the result of WE ARE UNITED nearly 30 years. It’s been both an honor and collaboration. And that collaboration is AND INSPIRED a privilege to work for Johnson & Johnson, driven by a diversity of ideas, individuals BY OUR CREDO, a company that touches the lives of over and disciplines – working together toward WHICH RINGS a billion people every day, around the a common goal. AS TRUE TODAY world. As I look at today’s health care AS IT DID WHEN landscape, it’s incredibly clear that the Today, more than ever, the world needs IT WAS WRITTEN pace of change has never been greater, leaders who are committed to working MORE THAN 70 or frankly, more exciting. together to help bring improved health YEARS AGO. and wellness to every person in every Today’s rapid change brings both corner of the globe. As the world’s largest opportunities and risks for any company and most broadly based health care in health care, and we are prepared company, we are uniquely positioned to help to address both. There are significant transform global health care; to shine a light challenges to overcome, but the tools, the on the most important issues we are facing; insights, the technologies, the innovations to collaborate across boundaries and – both evolutions and revolutions – all borders; to uncover scientific insights and combine to make today one of the most ideas; and to dedicate resources towards promising times for human health and for creating tomorrow’s breakthroughs. -

Partner with DNA
REA CH YOUR TARGE T A UDIENC E Partner with DNA 2017 CORPO R AT E MEMB ERSHIP P R OGRA M www.dnanurse.org Nurses Serving Nurses ABOUT for more than 30 years! The Dermatology Nurses’ Association ( DNA) is a professional nursing organization comprised of a diverse group of individuals committed to quality care through sharing knowledge and exper tise. For more than 30 years, DNA has promoted excellence in dermatological care, education and cer tification. The DNA is the longest-standing dermatology nursing association in the United States and Canada with more than 2,000 members covering all dermatologic specialties and with all levels of education and cer tification. Nurse Practitioner Highlights 76% Did you know that more than 25% of the DNA membership of dermatology consists of Nurse Practitioners (NPs) These highly educated professionals are practitioners are leaders in patient care in hospitals and private practice and have interest and knowledge in prescription DNA members medications, emerging medical technologies and research. because of the educational These valued professionals are also members of DNA’s Nurse Practitioner Society, which provides a forum for collaboration, and networking targeted education and networking among the DNA NPs. oppor tunities the The NP Forum, co-located with the DNA Annual Convention, is an intensive, two-day educational event highlighting the association provides. issues confronting today’s dermatology NPs. Membership by Professional Status Member Clinical Specialty DNA CORPORATE MEMBER HISTORY 3 Gen, LLC Celgene Ferndale Healthcare Inc. National Biological Corporation SkinMedica, Inc. 3M Health Care ConMed Corporation Galderma Laboratories Neocutis, Inc. Stiefel, a GSK Company AbbVie Coolibar, Inc. -

Swiss Biotech Success Stories Award Winner 2020 Actelion
Swiss Biotech report 2020_AWK-20-04-20_setup for perfect binding.qxp_Swiss Biotech report 2020_AWK_14 April 20/04/2020 15:42 Page 40 Swiss Biotech Success Stories award winner 2020 Actelion Body text or subhead here Actelion, a Janssen pharmaceutical company of Johnson & Johnson, is an industry leader in pulmonary hypertension. Established in 1997, the founding members set out to build a After being acquired by Johnson & Johnson in June 2017, company based on groundbreaking innovation and an Actelion leveraged its established global presence and unwavering commitment to patients. commercial strength bringing further resources, capabilities, and investment to change the future for people with PAH and After going public in April 2000, the company, led by CEO Jean- other types of cardiopulmonary diseases. At the time of its Paul Clozel M.D., launched its frst product, Tracleer (bosentan), acquisition, Actelion employed more than 2,600 people in over in the US in November 2001. 30 afliates, reaching more than 50 markets. Since the beginning, Actelion, in addition to its focus on Since the acquisition, the development of one of Actelion’s pulmonary hypertension, had built an active drug discovery and compounds, ponesimod, was transitioned to the Neuroscience development organization specializing in the areas of Therapeutic Area of Janssen. It is being investigated for the immunology, central nervous system disorder, and rare diseases, treatment of relapsing forms of multiple sclerosis in adults; last which now continues in Idorsia. In addition, Actelion created a year encouraging Phase III trial results were published. sales and marketing organization with global reach, leading to the launch of six new medicines, helping patients in the areas of Despite much progress, PAH has no cure and four out of 10 pulmonary hypertension, lipid storage disorders, and skin cancer. -

Selected Pharmaceuticals in Late Stage U.S. and E.U
SELECTED PHARMACEUTICALS IN LATE STAGE U.S. AND E.U. DEVELOPMENT OR REGISTRATION as of 04/17/18 Therapeutic Area Product Name Indication Sought U.S. Development Stage E.U. Development Stage XARELTO® (rivaroxaban) Reduce the risk of major adverse cardiac events (MACE) in patients with Chronic Heart Failure and Phase III Cardiovascular and significant Coronary Artery Disease (2) Metabolism Prevention of Symptomatic VTE and VTE-related death in high-risk, medically ill pts (2) Phase III Reduce the risk of MACE in patients with coronary or Peripheral Artery Disease (2) Filed 12/17 VTE prophylaxis in ambulatory cancer patients receiving chemo at high-risk for VTE (2) Phase III Infrainguinal revascular (2) Phase III Pediatric VTE (2) Phase III INVOKANA® (canagliflozin) Diabetic Nephropathy (2) Phase III Phase III Reduce the risk of death in type 2 diabetes with established, or risk for, cardiovascular disease. (CANVAS Filed 9/17 Filed 10/17 /CANVAS-R ) (2) Immunology TREMFYA® (guselkumab) Psoriatic arthritis Phase III Phase III Psoriasis Patient-Controlled Injector Filed 3/18 Phase III SIMPONI ARIA® (golimumab) Juvenile Idiopathic Arthritis (JIA) Phase III STELARA® (ustekinumab) Pediatric Psoriasis Phase III Phase III Ulcerative Colitis Phase III Phase III Infectious Diseases SYMTUZA™P Single tablet regimen for HIV in treatment naïve patients and treatment experienced patients (2) Filed 9/17 Approved 9/17 and Vaccines (darunavir/cobicistat/emtricitabine/ tenofovir alafenamide) JULUCA® (rilpivirine and Single tablet regimen for HIV in treatment -

95879-Medadnews-Nove
THE MAGAZINE OF PHARMACEUTICAL BUSINESS AND MARKETING • MEDADNEWS.COM • NOVEMBER 2012 • VOLUME 31 NUMBER 11 • $25 IN THIS ISSUE: TAKING iPad iPad MOBILE Ad-ventures in iPad 10 GLOBAL To successfully deploy marketing VI content in mobile ways in emerging markets, For the sixth year, Med Ad News has chosen three Pharmaceutical pharma needs to keep in mind the type of Marketing Ventures to Watch that could change the way technologies available pharmaceutical products are marketed and sold. and assess the true needs of patients and By Joshua Slatko [email protected] physicians. For the sixth time this past September, Med Ad News began anew its search for the future of pharmaceutical marketing. We sought out young companies, spin-off s, off erings, and ventures to profi le that are provid- ing the most innovative and interesting products, services, or marketing opportunities to pharmaceutical companies and the healthcare commu- nity. As ever, this year’s three profi lees came to their places in health- care from a variety of diff erent angles – one of them started somewhere completely diff erent – but all three are highly technology-based, and all three attempt to solve problems of communication – between the dif- ferent parts of pharmaceutical companies and their sales and marketing organizations, between companies and their external constituencies, and among patients themselves. Here are Med Ad News’ three Pharmaceutical Marketing Ventures to Watch for 2012. RISKY Appature Nexus BUSINESS 12 Appature Nexus is a cloud-based relationship marketing tool designed Shahani, now the company’s CEO, to meet the specifi c needs of marketing professionals in the healthcare came to the venture from the social industry.