Early Development of the Neural Plate, Neural Crest and Facial Region of Marsupials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

3 Embryology and Development
BIOL 6505 − INTRODUCTION TO FETAL MEDICINE 3. EMBRYOLOGY AND DEVELOPMENT Arlet G. Kurkchubasche, M.D. INTRODUCTION Embryology – the field of study that pertains to the developing organism/human Basic embryology –usually taught in the chronologic sequence of events. These events are the basis for understanding the congenital anomalies that we encounter in the fetus, and help explain the relationships to other organ system concerns. Below is a synopsis of some of the critical steps in embryogenesis from the anatomic rather than molecular basis. These concepts will be more intuitive and evident in conjunction with diagrams and animated sequences. This text is a synopsis of material provided in Langman’s Medical Embryology, 9th ed. First week – ovulation to fertilization to implantation Fertilization restores 1) the diploid number of chromosomes, 2) determines the chromosomal sex and 3) initiates cleavage. Cleavage of the fertilized ovum results in mitotic divisions generating blastomeres that form a 16-cell morula. The dense morula develops a central cavity and now forms the blastocyst, which restructures into 2 components. The inner cell mass forms the embryoblast and outer cell mass the trophoblast. Consequences for fetal management: Variances in cleavage, i.e. splitting of the zygote at various stages/locations - leads to monozygotic twinning with various relationships of the fetal membranes. Cleavage at later weeks will lead to conjoined twinning. Second week: the week of twos – marked by bilaminar germ disc formation. Commences with blastocyst partially embedded in endometrial stroma Trophoblast forms – 1) cytotrophoblast – mitotic cells that coalesce to form 2) syncytiotrophoblast – erodes into maternal tissues, forms lacunae which are critical to development of the uteroplacental circulation. -

Notch Signaling Regulates the Differentiation of Neural Crest From
ß 2014. Published by The Company of Biologists Ltd | Journal of Cell Science (2014) 127, 2083–2094 doi:10.1242/jcs.145755 RESEARCH ARTICLE Notch signaling regulates the differentiation of neural crest from human pluripotent stem cells Parinya Noisa1,2, Carina Lund2, Kartiek Kanduri3, Riikka Lund3, Harri La¨hdesma¨ki3, Riitta Lahesmaa3, Karolina Lundin2, Hataiwan Chokechuwattanalert2, Timo Otonkoski4,5, Timo Tuuri5,6,* and Taneli Raivio2,4,*,` ABSTRACT Kokta et al., 2013). Neural crest cells originate from neuroectoderm at the border between the neural plate and the Neural crest cells are specified at the border between the neural epiderm (Meulemans and Bronner-Fraser, 2004), and they are plate and the epiderm. They are capable of differentiating into marked by the expression of genes that are specific for the neural- various somatic cell types, including craniofacial and peripheral plate border, such as DLX5, MSX1, MSX2 and ZIC1. Later, during nerve tissues. Notch signaling plays important roles during the neural-tube folding process, neural crest cells remain within neurogenesis; however, its function during human neural crest the neural folds and subsequently localize inside the dorsal development is poorly understood. Here, we generated self- portion of the neural tube. These premigratory neural crest cells renewing premigratory neural-crest-like cells (pNCCs) from human express specifier genes, such as SNAIL (also known as SNAI1), pluripotent stem cells (hPSCs) and investigated the roles of Notch SLUG (also known as SNAI2), SOX10 and TWIST1 (LaBonne and signaling during neural crest differentiation. pNCCs expressed Bronner-Fraser, 2000; Mancilla and Mayor, 1996). Following the various neural-crest-specifier genes, including SLUG (also known formation of the neural tube, premigratory neural crest cells as SNAI2), SOX10 and TWIST1, and were able to differentiate into undergo an epithelial-to-mesenchymal transition (EMT) and most neural crest derivatives. -

Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease
Journal of Cardiovascular Development and Disease Review Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease Laura A. Dyer 1 and Sandra Rugonyi 2,* 1 Department of Biology, University of Portland, Portland, OR 97203, USA; [email protected] 2 Department of Biomedical Engineering, Oregon Health & Science University, Portland, OR 97239, USA * Correspondence: [email protected] Abstract: In congenital heart disease, the presence of structural defects affects blood flow in the heart and circulation. However, because the fetal circulation bypasses the lungs, fetuses with cyanotic heart defects can survive in utero but need prompt intervention to survive after birth. Tetralogy of Fallot and persistent truncus arteriosus are two of the most significant conotruncal heart defects. In both defects, blood access to the lungs is restricted or non-existent, and babies with these critical conditions need intervention right after birth. While there are known genetic mutations that lead to these critical heart defects, early perturbations in blood flow can independently lead to critical heart defects. In this paper, we start by comparing the fetal circulation with the neonatal and adult circulation, and reviewing how altered fetal blood flow can be used as a diagnostic tool to plan interventions. We then look at known factors that lead to tetralogy of Fallot and persistent truncus arteriosus: namely early perturbations in blood flow and mutations within VEGF-related pathways. The interplay between physical and genetic factors means that any one alteration can cause significant disruptions during development and underscore our need to better understand the effects of both blood flow and flow-responsive genes. -

The Migration of Neural Crest Cells and the Growth of Motor Axons Through the Rostral Half of the Chick Somite
/. Embryol. exp. Morph. 90, 437-455 (1985) 437 Printed in Great Britain © The Company of Biologists Limited 1985 The migration of neural crest cells and the growth of motor axons through the rostral half of the chick somite M. RICKMANN, J. W. FAWCETT The Salk Institute and The Clayton Foundation for Research, California division, P.O. Box 85800, San Diego, CA 92138, U.S.A. AND R. J. KEYNES Department of Anatomy, University of Cambridge, Downing St, Cambridge, CB2 3DY, U.K. SUMMARY We have studied the pathway of migration of neural crest cells through the somites of the developing chick embryo, using the monoclonal antibodies NC-1 and HNK-1 to stain them. Crest cells, as they migrate ventrally from the dorsal aspect of the neural tube, pass through the lateral part of the sclerotome, but only through that part of the sclerotome which lies in the rostral half of each somite. This migration pathway is almost identical to the path which pre- sumptive motor axons take when they grow out from the neural tube shortly after the onset of neural crest migration. In order to see whether the ventral root axons are guided along this pathway by neural crest cells, we surgically excised the neural crest from a series of embryos, and examined the pattern of axon outgrowth approximately 24 h later. In somites which contained no neural crest cells, ventral root axons were still found only in the rostral half of the somite, although axonal growth was slightly delayed. These axons were surrounded by sheath cells, which had presumably migrated out of the neural tube, to a point about 50 jan proximal to the growth cones. -

Defects of Embryonic Organogenesis Resulting from Targeted Disruption of the N-Myc Gene in the Mouse
Development 117, 1445-1455 (1993) 1445 Printed in Great Britain © The Company of Biologists Limited 1993 Defects of embryonic organogenesis resulting from targeted disruption of the N-myc gene in the mouse Shoji Sawai1, Akihiko Shimono1, Yoshio Wakamatsu1, Cynthia Palmes1, Kazunori Hanaoka2 and Hisato Kondoh1,* 1Department of Molecular Biology, School of Science, Nagoya University, Nagoya 464-01, Japan 2Division of Animal Models for Human Disease, National Institute of Neuroscience, NCNP, Ogawahigashi, Kodaira, Tokyo 187, Japan *Author for correspondence SUMMARY The highest expression of the N-myc gene occurs during death of the embryos. Analyses indicated that the embryonic organogenesis in the mouse ontogeny, with mutant limbs failed to develop distal structures and the the peak of expression around embryonic day 9.5. development of bronchi from the trachea was defective Homozygous N-myc-deficient mice, produced by germ- in the lungs. The latter defect was largely corrected by line transmission of a disrupted allele in ES cells, devel- addition of fetal calf serum to the culture medium, sug- oped normally to day 10.5, indicating dispensability of gesting that an activity missing in the mutant lung was N-myc expression in the earlier period, but later accu- replenished by a component of the serum. The pheno- mulated organogenic abnormalities and died around type of N-myc-deficient mutant embryos indicated day 11.5. The most notable abnormalities were found in requirement of the N-myc function in many instances of the limb bud, visceral organs (lung, stomach, liver and tissue interactions in organogenesis and also in cell- heart) and the central/peripheral nervous systems, and autonomous regulation of tissue maturation. -

Works Neuroembryology
Swarthmore College Works Biology Faculty Works Biology 1-1-2017 Neuroembryology D. Darnell Scott F. Gilbert Swarthmore College, [email protected] Follow this and additional works at: https://works.swarthmore.edu/fac-biology Part of the Biology Commons Let us know how access to these works benefits ouy Recommended Citation D. Darnell and Scott F. Gilbert. (2017). "Neuroembryology". Wiley Interdisciplinary Reviews: Developmental Biology. Volume 6, Issue 1. DOI: 10.1002/wdev.215 https://works.swarthmore.edu/fac-biology/493 This work is brought to you for free by Swarthmore College Libraries' Works. It has been accepted for inclusion in Biology Faculty Works by an authorized administrator of Works. For more information, please contact [email protected]. HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Wiley Interdiscip Manuscript Author Rev Dev Manuscript Author Biol. Author manuscript; available in PMC 2018 January 01. Published in final edited form as: Wiley Interdiscip Rev Dev Biol. 2017 January ; 6(1): . doi:10.1002/wdev.215. Neuroembryology Diana Darnell1 and Scott F. Gilbert2 1University of Arizona College of Medicine 2Swarthmore College and University of Helsinki Abstract How is it that some cells become neurons? And how is it that neurons become organized in the spinal cord and brain to allow us to walk and talk, to see, recall events in our lives, feel pain, keep our balance, and think? The cells that are specified to form the brain and spinal cord are originally located on the outside surface of the embryo. They loop inward to form the neural tube in a process called neurulation. -
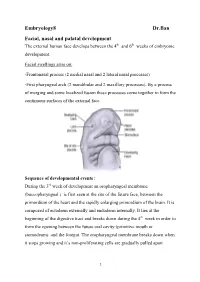
Embryology8 Dr.Ban Facial, Nasal and Palatal Development the External Human Face Develops Between the 4Th and 6Th Weeks of Embryonic Development
Embryology8 Dr.Ban Facial, nasal and palatal development The external human face develops between the 4th and 6th weeks of embryonic development. Facial swellings arise on: -Frontonasal process (2 medial nasal and 2 lateral nasal processes) -First pharyngeal arch (2 mandibular and 2 maxillary processes). By a process of merging and some localized fusion these processes come together to form the continuous surfaces of the external face. Sequence of developmental events : During the 3rd week of development an oropharyngeal membrane (buccopharyngeal ) is first seen at the site of the future face, between the primordium of the heart and the rapidly enlarging primordium of the brain. It is composed of ectoderm externally and endoderm internally. It lies at the beginning of the digestive tract and breaks down during the 4th week in order to form the opening between the future oral cavity (primitive mouth or stomodeum) and the foregut. The oropharyngeal membrane breaks down when it stops growing and it’s non-proliferating cells are gradually pulled apart 1 because they cannot fill the expanding area.The tissues around it expand very rapidly. The face develops from five primordia that appear in the fourth week: the frontonasal prominence, the two maxillary swellings, and the two mandibular swellings. The external face forms from two sources that surround the oropharyngeal membrane 1-Tissues of the frontonasal process that cover the forebrain, predominantly of neural crest origin. 2-The tissues of the first (or mandibular) pharyngeal arch, of mixed mesoderm and neural crest origin Face initially formed by 5 mesenchymal swellings ( prominences): Two mandibular prominences Two maxillary prominences Frontonasal prominence (midline structure is a single structure that is ventral to the forebrain. -

The Genetic Basis of Mammalian Neurulation
REVIEWS THE GENETIC BASIS OF MAMMALIAN NEURULATION Andrew J. Copp*, Nicholas D. E. Greene* and Jennifer N. Murdoch‡ More than 80 mutant mouse genes disrupt neurulation and allow an in-depth analysis of the underlying developmental mechanisms. Although many of the genetic mutants have been studied in only rudimentary detail, several molecular pathways can already be identified as crucial for normal neurulation. These include the planar cell-polarity pathway, which is required for the initiation of neural tube closure, and the sonic hedgehog signalling pathway that regulates neural plate bending. Mutant mice also offer an opportunity to unravel the mechanisms by which folic acid prevents neural tube defects, and to develop new therapies for folate-resistant defects. 6 ECTODERM Neurulation is a fundamental event of embryogenesis distinct locations in the brain and spinal cord .By The outer of the three that culminates in the formation of the neural tube, contrast, the mechanisms that underlie the forma- embryonic (germ) layers that which is the precursor of the brain and spinal cord. A tion, elevation and fusion of the neural folds have gives rise to the entire central region of specialized dorsal ECTODERM, the neural plate, remained elusive. nervous system, plus other organs and embryonic develops bilateral neural folds at its junction with sur- An opportunity has now arisen for an incisive analy- structures. face (non-neural) ectoderm. These folds elevate, come sis of neurulation mechanisms using the growing battery into contact (appose) in the midline and fuse to create of genetically targeted and other mutant mouse strains NEURAL CREST the neural tube, which, thereafter, becomes covered by in which NTDs form part of the mutant phenotype7.At A migratory cell population that future epidermal ectoderm. -

Late Effects of Retinoic Acid on Neural Crest and Aspects of Rhombomere Identity
Development 122, 783-793 (1996) 783 Printed in Great Britain © The Company of Biologists Limited 1996 DEV2020 Late effects of retinoic acid on neural crest and aspects of rhombomere identity Emily Gale1, Victoria Prince2, Andrew Lumsden3, Jon Clarke4, Nigel Holder1 and Malcolm Maden1 1Developmental Biology Research Centre, The Randall Institute, King’s College London, 26-29 Drury Lane, London WC2B 5RL, UK 2Department of Molecular Biology, Princeton University, Washington Road, Princeton, NJ 08544, USA 3MRC Brain Development Programme, Division of Anatomy and Cell Biology, UMDS, Guy’s Hospital, London SE1 9RT, UK 4Department of Anatomy and Developmental Biology, University College, Windeyer Building, Cleveland St., London W1P 6DB, UK SUMMARY We exposed st.10 chicks to retinoic acid (RA), both ment of the facial ganglion in addition to a mis-direction of globally, and locally to individual rhombomeres, to look at the extensions of its distal axons and a dramatic decrease its role in specification of various aspects of hindbrain in the number of contralateral vestibuloacoustic neurons derived morphology. Previous studies have looked at RA normally seen in r4. Only this r4-specific neuronal type is exposure at earlier stages, during axial specification. Stage affected in r4; the motor neuron projections seem normal 10 is the time of morphological segmentation of the in experimental animals. The specificity of this result, hindbrain and is just prior to neural crest migration. combined with the loss of Hoxb-1 expression in r4 and the Rhombomere 4 localised RA injections result in specific work by Krumlauf and co-workers showing gain of con- alterations of pathways some crest cells that normally tralateral neurons co-localised with ectopic Hoxb-1 migrate to sites of differentiation of neurogenic derivatives. -

Clonal Dispersion During Neural Tube Formation 4097 of Neuromeres
Development 126, 4095-4106 (1999) 4095 Printed in Great Britain © The Company of Biologists Limited 1999 DEV2458 Successive patterns of clonal cell dispersion in relation to neuromeric subdivision in the mouse neuroepithelium Luc Mathis1,*, Johan Sieur1, Octavian Voiculescu2, Patrick Charnay2 and Jean-François Nicolas1,‡ 1Unité de Biologie moléculaire du Développement, Institut Pasteur, 25, rue du Docteur Roux, 75724 Paris Cedex 15, France 2Unité INSERM 368, Ecole Normale Supérieure, 46 rue d’Ulm, 75230 Paris Cedex 05, France *Present address: Beckman Institute (139-74), California Institute of Technology, Pasadena, CA, 91125, USA ‡Author for correspondence (e-mail: [email protected]) Accepted 5 July; published on WWW 23 August 1999 SUMMARY We made use of the laacz procedure of single-cell labelling the AP and DV axis of the neural tube. A similar sequence to visualize clones labelled before neuromere formation, in of AP cell dispersion followed by an arrest of AP cell 12.5-day mouse embryos. This allowed us to deduce two dispersion, a preferential DV cell dispersion and then by a successive phases of cell dispersion in the formation of the coherent neuroepithelial growth, is also observed in the rhombencephalon: an initial anterior-posterior (AP) cell spinal cord and mesencephalon. This demonstrates that a dispersion, followed by an asymmetrical dorsoventral (DV) similar cascade of cell events occurs in these different cell distribution during which AP cell dispersion occurs in domains of the CNS. In the prosencephalon, differences in territories smaller than one rhombomere. We conclude that spatial constraints may explain the variability in the the general arrest of AP cell dispersion precedes the onset orientation of cell clusters. -

Semaphorin3a/Neuropilin-1 Signaling Acts As a Molecular Switch Regulating Neural Crest Migration During Cornea Development
Developmental Biology 336 (2009) 257–265 Contents lists available at ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Semaphorin3A/neuropilin-1 signaling acts as a molecular switch regulating neural crest migration during cornea development Peter Y. Lwigale a,⁎, Marianne Bronner-Fraser b a Department of Biochemistry and Cell Biology, MS 140, Rice University, P.O. Box 1892, Houston, TX 77251, USA b Division of Biology, 139-74, California Institute of Technology, Pasadena, CA 91125, USA article info abstract Article history: Cranial neural crest cells migrate into the periocular region and later contribute to various ocular tissues Received for publication 2 April 2009 including the cornea, ciliary body and iris. After reaching the eye, they initially pause before migrating over Revised 11 September 2009 the lens to form the cornea. Interestingly, removal of the lens leads to premature invasion and abnormal Accepted 6 October 2009 differentiation of the cornea. In exploring the molecular mechanisms underlying this effect, we find that Available online 13 October 2009 semaphorin3A (Sema3A) is expressed in the lens placode and epithelium continuously throughout eye development. Interestingly, neuropilin-1 (Npn-1) is expressed by periocular neural crest but down- Keywords: Semaphorin3A regulated, in a manner independent of the lens, by the subpopulation that migrates into the eye and gives Neuropilin-1 rise to the cornea endothelium and stroma. In contrast, Npn-1 expressing neural crest cells remain in the Neural crest periocular region and contribute to the anterior uvea and ocular blood vessels. Introduction of a peptide that Cornea inhibits Sema3A/Npn-1 signaling results in premature entry of neural crest cells over the lens that Lens phenocopies lens ablation. -

Dlx3b/4B Is Required for Early-Born but Not Later-Forming Sensory Hair Cells
© 2017. Published by The Company of Biologists Ltd | Biology Open (2017) 6, 1270-1278 doi:10.1242/bio.026211 RESEARCH ARTICLE Dlx3b/4b is required for early-born but not later-forming sensory hair cells during zebrafish inner ear development Simone Schwarzer, Sandra Spieß, Michael Brand and Stefan Hans* ABSTRACT complex arrangement of mechanosensory hair cells, nonsensory Morpholino-mediated knockdown has shown that the homeodomain supporting cells and sensory neurons (Barald and Kelley, 2004; Raft transcription factors Dlx3b and Dlx4b are essential for proper and Groves, 2015; Whitfield, 2015). Inner ear formation is a induction of the otic-epibranchial progenitor domain (OEPD), as multistep process initiated by the establishment of the preplacodal well as subsequent formation of sensory hair cells in the developing region, a zone of ectoderm running around the anterior border of the zebrafish inner ear. However, increasing use of reverse genetic neural plate containing precursors for all sensory placodes (Streit, approaches has revealed poor correlation between morpholino- 2007). The preplacodal region is further specified into a common induced and mutant phenotypes. Using CRISPR/Cas9-mediated otic-epibranchial progenitor domain (OEPD) that in zebrafish also mutagenesis, we generated a defined deletion eliminating the entire contains the progenitors of the anterior lateral line ganglion (Chen open reading frames of dlx3b and dlx4b (dlx3b/4b) and investigated a and Streit, 2013; McCarroll et al., 2012; Hans et al., 2013). potential phenotypic