Group Living, Breeding Behaviour and Territoriality in the Subdesert Mesite
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MADAGASCAR: the Wonders of the “8Th Continent” a Tropical Birding Custom Trip
MADAGASCAR: The Wonders of the “8th Continent” A Tropical Birding Custom Trip October 20—November 6, 2016 Guide: Ken Behrens All photos taken during this trip by Ken Behrens Annotated bird list by Jerry Connolly TOUR SUMMARY Madagascar has long been a core destination for Tropical Birding, and with the opening of a satellite office in the country several years ago, we further solidified our expertise in the “Eighth Continent.” This custom trip followed an itinerary similar to that of our main set-departure tour. Although this trip had a definite bird bias, it was really a general natural history tour. We took our time in observing and photographing whatever we could find, from lemurs to chameleons to bizarre invertebrates. Madagascar is rich in wonderful birds, and we enjoyed these to the fullest. But its mammals, reptiles, amphibians, and insects are just as wondrous and accessible, and a trip that ignored them would be sorely missing out. We also took time to enjoy the cultural riches of Madagascar, the small villages full of smiling children, the zebu carts which seem straight out of the Middle Ages, and the ingeniously engineered rice paddies. If you want to come to Madagascar and see it all… come with Tropical Birding! Madagascar is well known to pose some logistical challenges, especially in the form of the national airline Air Madagascar, but we enjoyed perfectly smooth sailing on this tour. We stayed in the most comfortable hotels available at each stop on the itinerary, including some that have just recently opened, and savored some remarkably good food, which many people rank as the best Madagascar Custom Tour October 20-November 6, 2016 they have ever had on any birding tour. -

Madagascar Highlights I 11 Th to 25 Th July 2011 (15 Days)
Madagascar Highlights I 11 th to 25 th July 2011 (15 days) Trip Report Trip report compiled by tour leader: Rainer Summers Tour Summary Sometimes referred to as the “laboratory of evolution”, Madagascar, the huge Indian Ocean island situated 500km off the coast of east Africa, has long attracted the attention of naturalists and travelling birders alike. Our winter tour, although a departure from the standard summer tours to the “Red Isle”, was very successful, and we managed to see a fantastic proportion of the amazing creatures that call Madagascar home. Trip Report RBT Madagascar Highlights I 2011 2 We began our first day with a visit to the Tsimbazaza Zoo, where despite the overcast weather we managed to find Eleonora’s Falcon, Mascarene Martin and the first of many Madagascar Buzzards, while our afternoon at Lake Alarobia proved to be most enjoyable, with large numbers of waterfowl including Knob-billed Duck and Red-billed Teal, Black and Dimorphic Egret, Malagasy Kingfisher and Madagascar Swamp Warbler, before enjoying a sumptuous dinner at our comfortable accommodations. The eastern rainforests of Madagascar harbour a rich assemblage of sought-after mammals and birds, and for this reason the forested zone in the vicinity of Perinet village formed the basis of our explorations for four days. Our time was divided between visits to the reserve at Perinet and the more distant Mantadia National Park, both offering good rainforest birding. Despite the less than optimal weather during our time in Perinet- Mantadia, our hard work and time in the field paid off, and we were rewarded with a mouth-watering selection of eastern rainforest endemics. -

Birding Madagascar 1-22 November 2018
Birding Madagascar 1-22 November 2018. Trip report compiled by Tomas Carlberg. 1 Front cover Daily log Red-capped Coua, sunbathing in Ankarafantsika National Park. Photo: Tomas Carlberg November 1st Some of us (TC, JN, and RN) flew Air France from Photos Arlanda, Stockholm at 06:00 to Paris, where we © All photos in this report: Tomas Carlberg. met OP (who flew from Gothenburg) and IF (flew For additional photos, see p. 30 ff. from Manchester). An 11 hrs flight took us to Antananarivo, where we landed just before Participants midnight. Once through after visa and passport control we met Zina at the airport. We stayed at IC Tomas Carlberg (Tour leader), Jonas Nordin, Hotel and fell asleep at 01:30. Sweden; Rolf Nordin, Sweden; Olof Persson, Sweden; Jesper Hornskov, Denmark; Eric November 2nd Schaumburg, Denmark; Hans Harrestrup Andersen, Woke up at 6, met the Danes (JH, ES, HW, and Denmark; Hans Wulffsberg, Denmark; Ian Fryer, UK HHA), and had breakfast. Changed c. 400 Euro each Serge “Zina” Raheritsiferana (organizer and driver), and got 1 540 000 ariary… Departure at 7:30 Fidson “Fidy” Albert Alberto (guide), and Lala. heading north towards Ankarafantsika NP. Saw a male Malagasy Harrier c. 16 km south of Ankazobe Correspondence (-18.45915, 47.160156), so stopped for birding [email protected] (Tomas Carlberg) there 9:45-10:05. Stop at 11:40 to buy sandwiches for lunch. Lunch with birding 12:55-13:15. Long Tour organizers transport today… Stopped for birding at bridge Serge “Zina” Raheritsiferana (Zina-Go Travel), over Betsiboka River 16:30-17:30; highlight here Stig Holmstedt. -

Madagascar: the 8Th Continent with Naturalist Journeys & Caligo Ventures Nov
Madagascar: The 8th Continent With Naturalist Journeys & Caligo Ventures Nov. 26 – Dec. 10, 2018 866.900.1146 800.426.7781 520.558.1146 [email protected] www.naturalistjourneys.com or find us on Facebook at Naturalist Journeys, LLC Naturalist Journeys, LLC / Caligo Ventures PO Box 16545 Portal, AZ 85632 PH: 520.558.1146 / 800.426.7781 Fax 650.471.7667 naturalistjourneys.com / caligo.com [email protected] / [email protected] Madagascar: The 8th Continent With Naturalist Journeys & Caligo Ventures Isolated from any continental landmass since the Cretaceous period, Madagascar has drifted through the Indian Ocean, following its own evolutionary course, having only five major terrestrial animal colonization events since the time of the dinosaurs. The result is an island where every land mammal is endemic, as are nearly half the bird species. Reptiles are well represented as well, like chameleons, and day and leaf-tailed geckos. The uniqueness of this island’s fauna makes it one of the world’s great destinations for the birdwatcher and naturalist, alike. Our tour features both birds and mammals. We focus on Madagascar’s most iconic and charismatic bird species (we hope to see over 95% of the endemics), as well as the Island's other oddities, like endearing lemurs and strikingly bizarre chameleons. We also focus on the Island’s geology and geography with resulting various habitats ― from the spiny forests of Ifaty with its towering baobabs and other-worldly Didierea octopus trees, to the verdant rainforests of Andasibe-Mantadia -

University of Victoria Department of Biology INCREASES IN
University of Victoria Department of Biology INCREASES IN CHARCOAL PRODUCTION EFFICIENCY AND THE IMPLEMENTATION OF A SUSTAINABLE CHARCOAL SUPPLY CHAIN TO THE CITY OF TOLIARA IN SOUTHWESTERN MADAGASCAR WORK TERM REPORT In partial fulfillment of the requirements of the Biology Co-op Program Winter 2010 Work Term 1 By Julie Bremner WWF Explore International Youth Volunteer Performed at : WWF Madagascar and West Indian Ocean Programme Office Ankilimalinika, Madagascar Job Supervisor : Rina Andrianarivony Fuel wood Project Officer 1 TABLE OF CONTENTS ABSTRACT 2 INTRODUCTION 3 DISCUSSION 8 CONCLUSION 16 WORKS CITED 18 MAPS 20 2 ABSTRACT The Spiny Forest Ecoregion of Southwestern Madagascar is a zone of tremendous biodiversity and endemism. It is of key importance to the subsistence lives of villagers in the region and the urban population of Toliara that increasingly depends on forest fuel wood resources for their daily energy needs. Prolonged drought conditions in the area have led to increasing demands on the forest while villagers switch from farming to charcoal production as a means of earning a living. Urban population growth and resultant fuel wood demand increase has further exacerbated the deforestation of the spiny forest, which is currently exhibiting the highest rate of deforestation in Madagascar. WWF has stepped in to attempt to mitigate future forest loss through the establishment of the Synergy Energy Environment in the South West (SEESO) project. SEESO has as its goal the establishment of a sustainable fuel wood supply chain to the city of Toliara originating from the Atsimo-Andrefana region. The project is encouraging the adoption of a more efficient charcoal production technique, the plantation of trees for future charcoal production and the implementation of a system of regulations and governing bodies that will ensure the prolonged sustainability of the region’s forest resources. -

MADAGASCAR TRIP REPORT Aug.-‐Sept 2012 John Clark
MADAGASCAR TRIP REPORT Aug.-Sept 2012 John Clark ([email protected]) Our London friends, Dick and Liz Turner, Mary Ward-Jackson and I spent almost 4 weeks in Madagascar. Our primary focus was Birds, But we were also interested in nature more Broadly and culture. The tour was excellently prepared By our guide, Fanomezantsoa Andrianirina (Fano) – who was a perfect guide as well as Being great fun to travel with. The trip was excellent and we ended up seeing 122 of the endemic (and endemic Breeding) Birds of Madagascar, plus 54 non-endemics. Fano was not only an excellent Bird-guide himself, But he had lined up local guides in most of the locations – most of whom were terrific (especially, perhaps, Jaqui in Ampijoroa). Fano is doing much to help develop these local guides as more experienced and confident bird-guides in their own right. The logistics and places to stay were excellent – well, as excellent as an inevitaBle dependence on Madagascar Air permits! (They don’t call it Mad. Air for nothing; it is quite the worst airline I have ever had to use!). Fano’s drivers were also terrific (and keen budding birders!) So our main advice, for those planning a birding (or indeed broader nature/wildlife) trip to Mad. is to use Fano if at all possible. He was totally professional, accurate, dogged, scientifically knowledgeaBle about the Bird, mammals and other species and became a good friend. He can Be contacted By email on [email protected], phone: (+261)32 02 017 91 or website: www.madagascar-funtourguide.com If you want more info on the trip, please email me, and if you’d like to see some of our photos go to: https://picasaweb.google.com/104472367063381721824/Madagascar2012?authkey=Gv1sRgcJH0nYK-wenN9AE# Itinerary Aug. -

Reference File
References added since publication of 2007 CRC Handbook of Avian Body Masses Abadie, K. B., J. Pérez Z., and M. Valverde. 2006. Primer reporte de colonias del Martín Peruano Progne murphyi. Cotinga 24:99-101. Ackerman, J. T., J. Y. Takekawa, J. D. Bluso, J. L. Yee, and C. A. Eagles-Smith. 2008. Gender identification of Caspian Terns using external morphology and discriminant function analysis. Wilson Journal of Ornithology 120:378-383. Alarcos, S., C. de la Cruz, E. Solís, J. Valencia, and M. J. García-Baquero. 2007. Sex determination of Iberian Azure-winged Magpies Cyanopica cyanus cooki by discriminant analysis of external measurements. Ringing & Migration 23:211-216. Albayrak, T., A. Besnard, and A. Erdoğan. 2011. Morphometric variation and population relationships of Krüeper’s Nuthatch (Sitta krueperi) in Turkey. Wilson Journal of Ornithology 123:734-740. Aleixo, A., C. E. B. Portes, A. Whittaker, J. D. Weckstein, L. Pedreira Gonzaga, K. J. Zimmer, C. C. Ribas, and J. M. Bates. 2013. Molecular systematics and taxonomic revision of the Curve-billed Scythebill complex (Campylorhamphus procurvoides: Dendrocolaptidae), with description of a new species from western Amazonian Brazil. Pp. 253-257, In: del Hoyo, J., A Elliott, J. Sargatal, and D.A. Christie (eds). Handbook of the birds of the world. Special volume: new species and global index. Lynx Edicions, Barcelona, Spain. Volume 1. Alfano, A. 2014. Pygmy Nightjar (Nyctopolus hirundinaeus). Neotropical Birds Online (T.S. Schulenberg, ed.). Cornell Laboratory of Ornithology, Ithaca, NY. Alvarenga, H. M. F., E. Höfling, and L. F. Silveira. 2002. Notharchus swainsoni (Gray, 1846) é uma espécie válida. -

MADAGASCAR: the Wonders of the “8Th Continent” a Tropical Birding Set Departure
MADAGASCAR: The Wonders of the “8th Continent” A Tropical Birding Set Departure November 3—28, 2013 Guide: Ken Behrens All photos taken during this trip. All photos by Ken Behrens unless noted otherwise. TOUR SUMMARY Madagascar has long been a core destination for Tropical Birding, and with last year’s opening of a satellite office in the country, we have further solidified our expertise in the “Eighth Continent.” This was another highly successful set-departure tour to this special island. It included both the Northwestern Endemics Pre-Trip at the start and the Helmet Vanga extension to the Masoala Peninsula at the end. Although Madagascar poses some logistical challenges, especially in the form of the national airline Air Madagascar, we had no problems on this tour, not even a single delayed flight! The birding was great, with 196 species recorded, including almost all of the island’s endemic birds. As usual, the highlight was seeing all five of the incredible ground-rollers, from the roadrunner-like Long-tailed of the spiny forest to the wonderful rainforest-dwelling Scaly. There was a strong cast of vangas, including Helmet, Bernier’s, and Sickle-billed. In fact, we saw every member of the family save the mysterious Red-tailed Newtonia which is only regularly seen in the far south. As normal, the couas were also a favorite. From the shy and beautiful Red-breasted of Madagascar Set Departure Tour Nov. 3-28, 2013 the eastern rainforest to the huge Giant Coua of the dry western forest, we were looking for and at couas virtually every day! The bizarre mesites form a Malagasy endemic family, and we had superb extended views of all three members of the family. -
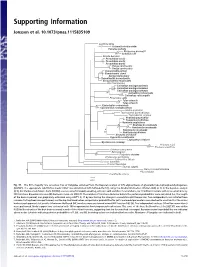
Supporting Information
Supporting Information Jønsson et al. 10.1073/pnas.1115835109 Fig. S1. The 50% majority rule consensus tree of Vangidae obtained from the Bayesian analysis of 375 aligned bases of glyceraldehyde-3-phosphodehydrogenase (GAPDH). The appropriate substitution model TIM+Γ was determined with MrModeltest (1), using the Akaike information criterion (AIC) (2, 3). In the Bayesian analysis (4, 5), the Markov chain Monte Carlo (MCMC) was run using Metropolis coupling, with one cold and three heated chains, for 10 million iterations with trees sampled every 500 iterations. Bayesian inference (BI) harmonic mean −ln 2962.70. The number of iterations discarded before the posterior probabilities were calculated (i.e., the length of the burn-in period) was graphically estimated using AWTY (6, 7) by monitoring the change in cumulative split frequencies. Two independent runs initiated from random starting trees were performed, and the log-likelihood values and posterior probabilities for splits and model parameters were checked to ascertain that the chains had reached apparent stationarity. Maximum-likelihood (ML) analyses were performed using GARLI version 0.95 (8). Five independent analyses (20 million generations) were performed. Nodal support was evaluated with 100 nonparametric bootstrap pseudoreplications. The score of the best-likelihood tree (−ln 2827.81) was within 0.05 likelihood units of the best tree recovered in each of the other four runs, suggesting that the five runs had converged. Bayesian posterior probabilities are indicated above nodes (asterisks indicate posterior probabilities of 1.00), and ML bootstrap values are indicated below nodes. Members of Vangidae are indicated in bold. 1. Nylander JAA (2004) MrModeltest (Uppsala University, Uppsala) (http://www.abc.se/∼nylander), Version 2. -

Wqfm: Statistically Consistent Genome-Scale Species Tree Estimation from Weighted Quartets
bioRxiv preprint doi: https://doi.org/10.1101/2020.11.30.403352; this version posted December 1, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. wQFM: Statistically Consistent Genome-scale Species Tree Estimation from Weighted Quartets Mahim Mahbub1;y, Zahin Wahab1;y, Rezwana Reaz1;y, M. Saifur Rahman1, and Md. Shamsuzzoha Bayzid1,* 1Department of Computer Science and Engineering Bangladesh University of Engineering and Technology Dhaka-1205, Bangladesh yThese authors contributed equally to this work *Corresponding author: shams [email protected] Abstract Motivation: Species tree estimation from genes sampled from throughout the whole genome is complicated due to the gene tree-species tree discordance. Incom- plete lineage sorting (ILS) is one of the most frequent causes for this discordance, where alleles can coexist in populations for periods that may span several speciation events. Quartet-based summary methods for estimating species trees from a collec- tion of gene trees are becoming popular due to their high accuracy and statistical guarantee under ILS. Generating quartets with appropriate weights, where weights correspond to the relative importance of quartets, and subsequently amalgamating the weighted quartets to infer a single coherent species tree allows for a statistically consistent way of estimating species trees. However, handling weighted quartets is challenging. Results: We propose wQFM, a highly accurate method for species tree es- timation from multi-locus data, by extending the quartet FM (QFM) algorithm to a weighted setting. -

Birds of Madagascar and Their Conservation
Birds of Madagascar and Their Conservation byMichael S. Putnam Department ofZoology University of Wisconsin tl Madison, Wisconsin I 100 plus Ecstatic Testimonials Striding up a steep hillside with the Council for Bird Preservation (Collar loud whisper of a rushing stream in and Stuart, 1985), 28 species of Warm, nurturing foods. the background, I stepped into a Malagasy birds are threatened and 14 mist-net lane I had cut a week before. species are considered as near Cook monthly, As I entered the clearing, a medium threatened. These species represent freeze in packets, sized brown bird squawked and flew between one-fifth and one-third of off from eye-level. Carefully search the island's endemic bird species. serve in seconds. ing the nearby vegetation, I became one of a lucky handful of foreigners The primary threats to Madagas At fine stores, orcall... to ever find a nest of the Brown car's birds today, habitat loss and Mesite (Mesitornis unicolor), a rare overhunting, have already eliminated 1(800) BIRD YUM forest-dwelling relative of rails. The many unique Malagasy creatures. 1 (800) 247-3986 large egg, delicately colored in sal Since people first arrived on Mada mon with liver-colored spots, rested gascar 1500 to 2000 years ago, much 13330 Bessemer Street precariously in a frail, dove-like nest of the island has been deforested, Van Nuys, CA91401-3000 positioned at the end of a sloping leaving the red lateritic soil exposed (818) 997-0598 sapling. This encounter with the and eroding, with little chance for Brown Mesite is just one of many forest regeneration. -

National Parks in Madagascar
NATIONAL PARKS IN MADAGASCAR Madagascar’s National Parks are divided into 4 parts: Deciduous Forest, Eastern Rain Forests, Island and Coastal and Spiny Forests and in total have about 28 National Parks across the island worth visiting DECIDUOUS FOREST 1. Zombitse-Vohibasia National Park Normally included as a short stop between Isalo and Tulear, the forest of Zombitse- Vohibasia is in a transition zone between dry deciduous and spiny forest habitats. Birders will appreciate seeing Appert’s greenbul, found nowhere else, giant, Coquerel’s and olive-capped couas, as well as various vanga species. 2. Andringitra National Park A spectacular and biodiverse reserve with an altitude range of 500 to 2,658 metres and mountainous outcrops of ancient Precambrian granite, waterfalls, lakes and unusual vegetation. Pic Boby, Madagascar’s second highest mountain, is a tough climb, but there are other less challenging trails through some magnificent scenery and habitats, including lowland forest, high humid tropical forest, sclerophyll and bamboo forest, bush and heathland. It has much endemic flora and over 100 species of birds, as well as over 50 mammal species including mountain-adapted ring tailed lemurs with thick coats. The climate ranges from humid tropical in the lowland rainforests to below freezing at altitude – indeed, it is the only place in Madagascar where snow has been recorded. 3. Ankarafantsika National Park ( Ampijoroa) This prime example of tropical dry deciduous forest, combined with a lake harbouring Nile crocodiles and endangered Madagascar fish eagles, contains many other rare, endemic birds including Van Dam’s vanga, sickle-billed vanga and red-capped coua.