Stress Transforms Lateral Habenula Reward Responses Into Punishment Signals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Scientific Program
Program 17th Chinese Biophysics Congress Scientific Program August 2nd (Friday) Registration Time Venue Schedule Lobby of Society Hill International 08:30-18:00 Conference Centre Registration Hotel Forum for BSC Journals Time Venue Schedule Grand Congress 13:30-15:30 Forum for BSC Journals Room 1 科普讲座 Time Venue Schedule Grand Congress 14:00-15:40 科普讲座 Room 2 Opening Ceremony Time Venue Schedule Grand Congress 16:30-17:00 Opening Ceremony Room 5,6 Plenary Lecture Venue: Grand Congress Room 5,6 Time Speaker Title /Affiliation Page Progress in Diagnosis & Treatment of Liver Cancer 17:00-17:45 Hongyang Wang (National Center for Liver Cancer, China) 28 Structure and function of the innate immune receptor RIG-I: 17:45-18:30 Anna Marie Pyle Strategies for signaling and for viral RNA specificity. 29 (Howard Hughes Medical Institute, Yale University) Welcome Reception Time Venue Schedule 18:00-20:00 Romano Restaurant Welcome Reception - 5 - Program 17th Chinese Biophysics Congress rd August 3 (Saturday) S1-1 结构与计算生物学Ⅰ Time: August 3rd. 8:30-11:50 Venue: Grand Congress Room 1 No Time Speaker Title / Affiliation Page 8:30-11:50 Chairs: Ruiming Xu, Jooyoung Lee Exploring the Folding Mechanism of Small Proteins GB1 1 8:30-9:05 Jooyoung Lee and LB1 30 (Korea Institute for Advanced Study, Korea) 生物分子的结构与动力学及其功能机理的分子动力学模 2 9:05-9:30 Guohui Li 拟理论方法发展与应用研究 31 (Dalian Institute of Chemical Physics, CAS, China) 3D Structural Visualization of the Long Non-coding 3 9:30-9:55 Xianyang Fang Subgenomic Flavivirus RNAs in Solution 32 (Tsinghua University, China) -
Nerve Growth Factor & Related Neurotrophic
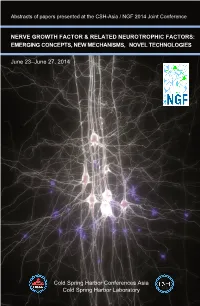
Abstracts of papers presented at the CSH-Asia / NGF 2014 Joint Conference NERVE GROWTH FACTOR & RELATED NEUROTROPHIC FACTORS: EMERGING CONCEPTS, NEW MECHANISMS, NOVEL TECHNOLOGIES June 23–June 27, 2014 Cold Spring Harbor Conferences Asia Cold Spring Harbor Laboratory Abstracts of papers presented at the CSH-Asia / NGF 2014 Joint Conference NERVE GROWTH FACTOR & RELATED NEUROTROPHIC FACTORS: EMERGING CONCEPTS, NEW MECHANISMS, NOVEL TECHNOLOGIES June 23–June 27, 2014 Arranged by Bai Lu, Tsinghua University Medical School, Beijing, China Tadaharu Tsumoto, RIKEN Brain Science Institute, Wako, Japan Moses Chao, Skirball Institute NYU, New York, USA Brian B. Rudkin, CNRS/Ecole Normale Supérieure de Lyon, France & East China Normal University, Shanghai, China Regional Advisory Committee Mu-ming Poo, Institute of Neuroscience, CAS, & UC Berkley, China/USA Nancy Ip, Hong Kong University of Science and Technology, Hong Kong Ralph Bradshaw, University of California San Francisco, USA Robert Rush, Flinders University, Australia Chonggang Yuan, East China Normal University, Shanghai, China Cold Spring Harbor Conferences Asia Cold Spring Harbor Laboratory Cold Spring Harbor Asia conferences in 2014 are generously supported in part by funds provided by our Genome Level Sponsor DNA Level Sponsor ____________________________________________________________ Cover: This image is a 3-dimensional reconstruction of cells (the 7 white cells). The blue dots indicate synapses between two cells. The synapses are points at which neurotransmitters (chemical messengers) -
V PROGRAM MONDAY, April 15—7:00 PM SESSION 1 KEYNOTE
PROGRAM MONDAY, April 15—7:00 PM SESSION 1 KEYNOTE SESSION Chairperson: Hailan Hu, Zhejiang University, Hangzhou, China Macaque models for neurobiology and biomedical research Mu-ming Poo [35’+10’] Presenter affiliation: Chinese Academy of Sciences, Shanghai, China. 1 Impact of age on neural circuits critical to memory Carol A. Barnes [35’+10’] Presenter affiliation: University of Arizona, Tucson, Arizona. 2 TUESDAY, April 16—9:00 AM SESSION 2 MOLECULAR, CELLULAR AND CIRCUIT MECHANISM OF MEMORY Chairperson: John O'Keefe, University College London, United Kingdom Genetic manipulations of CCR5 and the multifaceted molecular, cellular and circuit mechanisms of cognitive enhancement—A cautionary tale Miou Zhou, Yang Shen, Mary T. Joy, Einor B. Assayag, Dalia Shabashov-Stone, Marcela Arenas, Ayal Lavi, Shan Huang, Tawnie Silva, Nikita Thareja, Amos D. Korczyn, Sigal Liraz-Zaltsman, Efrat L. Kesner, Natan M. Bornstein, Esther Shohami, Stanley T. Carmichael, Alcino J. Silva [20’+10’] Presenter affiliation: UCLA, Los Angeles, California. 3 Mechanisms of experience-dependent hippocampal representations Jeff Magee [20’+10’] Presenter affiliation: HHMI- Baylor Coll of Medicine, Houston, Texas. 4 v Lamina-specific AMPA receptor dynamics following visual deprivation in vivo Han L. Tan, Richard H. Roth, Austin R. Graves, Robert H. Cudmore, Richard L. Huganir [10+5’] Presenter affiliation: Johns Hopkins University School of Medicine, Baltimore, Maryland. 5 Activity-dependent and projection-specific effects of astrocytes on plasticity and memory Inbal Goshen [20’+10’] Presenter affiliation: The Hebrew University, Jerusalem, Israel. 6 Astrocyte signaling within striatal microcircuits in vivo Baljit S. Khakh [20’+10’] Presenter affiliation: UCLA, Los Angeles, California. 7 Optogenetic control of cellular processes in the mouse brain Won Do Heo [20’+10’] Presenter affiliation: Korea Advanced Institute of Science and Technology(KAIST), Daejeon, South Korea; Institute for Basic Science (IBS), Daejeon, South Korea. -

Detailed Program
PROGRAM MONDAY, April 12—7:30 PM INTRODUCTION David Stewart Cold Spring Harbor Laboratory Maoyen Chi Cold Spring Harbor Asia SESSION 1 KEYNOTE SPEAKERS KEYNOTE I Introduction by Mu-ming Poo Linda Buck Howard Hughes Medical Institute Fred Hutchinson Cancer Research Center “Deconstructing smell” 1 KEYNOTE II Introduction by Z. Josh Huang Karl Deisseroth Stanford University “Optogenetics—Development and application” 2 v TUESDAY, April 13—9:00 AM SESSION 2 NEUROGENESIS Chairperson: Z. Josh Huang, Cold Spring Harbor Laboratory, USA Looking into the developing zebrafish retina William A. Harris. Presenter affiliation: Cambridge University, Cambridge, United Kingdom. 3 Creating the cortex, assembling the amygdala Shubha Tole. Presenter affiliation: Tata Institute of Fundamental Research, Mumbai, India. 4 Temporal regulation of neural stem cell fate in the developing mouse neocortex Yukiko Gotoh, Masafumi Tsuboi, Yusuke Kishi, Nao Suzki, Yusuke Hirabayashi. Presenter affiliation: University of Tokyo, Tokyo, Japan. 5 Regulation of midline glial development by nuclear factor one genes in the cerebral cortex Linda J. Richards. Presenter affiliation: The University of Queensland, Brisbane, Australia. 6 Stimulation of latent, neurogenic precursor pools in the hippocampus by synaptic activity Perry F. Bartlett, Dhanisha Jhaveri, Tara L. Walker. Presenter affiliation: The University of Queensland, Brisbane, Australia. 7 Neurogenesis—Its implication in and application for mental diseases Noriko Osumi. Presenter affiliation: Tohoku University Graduate -

Francis Crick Symposium
COLD SPRING HARBOR ASIA Francis Crick Symposium- Transforming Neurosciences: Organizers (Speaker, Affiliation, Country/Region) Questions & Experiments Hailan Hu Zhejiang University, CHINA Maiken Nedergaard University of Rochester Medical Center, USA John O’Keefe University College London, UK Hee-Sup Shin Institute for Basic Science, SOUTH KOREA Alcino Silva Suzhou, China University of California, Los Angeles, USA April 15-19, 2019 Abstract deadline: February 22, 2019 Keynote Speakers Major Topics (Speaker, Affiliation, Country/Region) Mu-Ming Poo, Institute of Neuroscience, CAS, CHINA Molecular, cellular and circuit mechanisms Carol Barnes, University of Arizona, USA of memory Invited Speakers Social neuroscience (Speaker, Affiliation, Country/Region) Instinct behavior Camilla Bellone, University of Geneva, SWITZERLAND Mauro Costa-Mattioli, Baylor College of Medicine, USA New technology Inbal Goshen, The Hebrew University, ISRAEL Hippocampal formation Won Do Heo, KAIST, SOUTH KOREA Neuroglial signaling Hailan Hu, Zhejiang University, CHINA Baljit Khakh, University of California, Los Angeles, USA Anatol Kreitzer, University of California, San Francisco, USA Julija Krupic, University of Cambridge, UK Stephan Lammel Fellowships , University of California, Berkeley, USA C. Justin Lee, Korea Institute of Science & Technology, SOUTH KOREA Bo Li, Cold Spring Harbor Laboratory, USA We are eager to have as many young people as possible attend Yulong Li since they are likely to benefit most from this meeting. A number of , Peking University, CHINA presentations by graduate students and postdocs in this conference Christian Lüscher, University of Geneva, SWITZERLAND will be selected for major fellowship awards. Jeffrey Magee, Baylor College of Medicine, USA Robert Malenka, Stanford University School of Medicine, USA Maiken Nedergaard, University of Rochester, USA Cold Spring Harbor Asia (CSHA) is the Asia-Pacific subsidiary of New York based Hitoshi Okamoto, RIKEN Brain Science Institute, JAPAN Cold Spring Harbor Laboratory (CSHL). -

V PROGRAM TUESDAY, September 25—7:00 PM SESSION 1 PLENARY TALKS Chairperson: Kazuo Emoto, the University of Tokyo, Tokyo, Japa
PROGRAM TUESDAY, September 25—7:00 PM SESSION 1 PLENARY TALKS Chairperson: Kazuo Emoto, The University of Tokyo, Tokyo, Japan Beyond memory circuit—Brain-wide network of cognitive memory and metamemory in the primate Yasushi Miyashita [35’+10’] Presenter affiliation: RIKEN, Wako, Japan. 1 Connections and communications between distributed neural circuits Liqun Luo [35’+10’] Presenter affiliation: Stanford University, Stanford, California. 2 WEDNESDAY, September 26—9:00 AM SESSION 2 CIRCUIT FORMATION, DEGENERATION, AND REGENERATION Chairperson: Takeshi Imai, Kyushu University, Fukuoka, Japan ADF/Cofilin-mediated actin turnover promotes axon regeneration in the adult CNS Frank Bradke [20+10’] Presenter affiliation: German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany. 3 Molecular and cellular basis for dendrite regeneration and regeneration in Drosophila Kazuo Emoto [20+10’] Presenter affiliation: The University of Tokyo, Tokyo, Japan. 4 Coffee / Tea Break v Experience-dependent regulation of structural and functional plasticity in Xenopus optic tectal neurons Hollis T. Cline, Hai-yan He, Wanhua Shen [20+10’] Presenter affiliation: The Scripps Research Institute, La Jolla, California. 5 Function and connectivity of optic flow processing circuit in the zebrafish pretectum Fabian Svara, Anna Kramer, Winfried Denk, Herwig Baier, Fumi Kubo [10’+3’] Presenter affiliation: Max Planck Institute of Neurobiology, Martinsried, Germany; National Institute of Genetics, Mishima, Japan. 6 Building a rat olfactory system in the mouse using interspecies chimeras Benjamin T. Throesch, Jun Wu, Masahiro Sakurai, Zhuhao Wu, Pavel Osten, Juan Carlos Izpisua Belmonte, Kristin K. Baldwin [10’+3’] Presenter affiliation: University of California San Diego, La Jolla, California; The Scripps Research Institute, La Jolla, California. 7 WEDNESDAY, September 26—2:00 PM SESSION 3 EMOTION, SLEEP, AND SOCIAL BEHAVIOR Chairperson: Yoko Yazaki-Sugiyama, OIST Graduate University, Okinawa, Japan Nirao Shah [20’+10’] Presenter affiliation: Stanford University, Stanford, California.