CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BTM-Layout Sub-Register Office
2017-18 Guidance value for the Immovable Properties coming under the jurisdiction of BTM-Layout Sub-Register Office. Residential Sites approved by Apartments/Flats/Villament constructed Competent Authority/ Local on Residential Sites approved by Organization Competent Authority/Local SI NO Hobli/Village/Ward/Road/Area Name Organization (Rupees per Square Meter For Super (Rupees per Squre Meter) Built up Area) 1 2 3 4 Industrial Area Nayanappa Shetty Palya and 1 65100 Bannerghatta Main Road 2 Tank Bund Road, N.S.Palya 52100 N.S. Palya Village area and Corporation 3 53290 area Main Roads N.S. Palya Village area and Corporation 4 47370 area Cross Roads 5 Janata Colony, N.S.Palya 43810 6 Society Layout area approved by BDA 59210 7 BTM 2nd Stage , N.S.Palya 59200 8 Bank Layout, N.S.Palya 59200 Dollars Schemes,BTM 2nd 9 91200 Stage (N.S.Palya and Bilekahalli) Mico Employees HBCS Layout/Mico Layout 10 68680 Main Roads Mico Employees HBCS Layout/Mico Layout 11 62170 Cross Roads 16th Main Road, Metropolitan, HBCS Layout 12 to 100 ft Ring Road (Mico Layout, BTM 2nd 87030 stage) 16th Main Road ( EWS Schemes to 13 Mahadeshwara Layout, N S Palya, Tankbund 62170 60 ft Road) 14 Mahadeshwara Layout Main Roads 49730 15 Mahadeshwara Layout Cross Roads. 43810 16 bharath house building co-operative society 59200 17 Abbayappa Layout, N S Palya, Main Roads 46180 18 Abbayappa Layout, N S Palya, cross Roads 37890 19 Reva Paradise 44800 20 Anand Apartment 44800 21 Tirumala Splendor 44800 22 Cholarenkil Lake view apartment 37100 23 Dream Sarovar apartment 37100 -

Svamitva-Emerald-Square-5041.Pdf
ENGINEERING TRUST, CRAFTING SATISFACTION DREAMS WITHIN YOUR REACH An ideal lifestyle is deeply ingrained in the homes we choose to live in. Keeping with the name Svamitva Emerald Square, the project aspires to capture the essence of an Emerald - a stone synonymous with tranquillity. Svamitva hopes to provide its residents with a home that is not only vaastu compliant but brings out the positivity, creativity, balance and confidence in its inhabitants - the characteristic qualities of an emerald. True to the qualities of its eponymous gemstone, Svamitva Emerald Square hopes to create an ideal lifestyle that inspires positivity in every resident. Despite the bustling surroundings, the calmness of the space makes for a refreshing change from hectic urban life. Emerald Square, situated in nature with a close proximity to the urban areas is the ideal home POSITIVITY for your family. AN OASIS OF TRANQUILITY. THE PILLARS TO YOUR DREAM HOME Svamitva Emerald Square is a 4 towered residential community located on Bommasandra - Jigani Link Road and close to Electronic City, Bengaluru. This project has been designed to fulfill every wish of its residence. The project houses 1, 2 and 3 BHK flats and every high - end amenity at affordable prices and also features various other conveniences that would make the life of its residents simple and idyllic. Svamitva Emerald Square is truly a place where your dreams are within your reach. Located a stone’s throw away from Bengaluru’s burgeoning IT Hub, Svamitva Emerald Square has been developed in an area that is peaceful and perfect for residents who value their solitude. -

Government of Karnataka Revenue Village, Habitation Wise
Government of Karnataka O/o Commissioner for Public Instruction, Nrupatunga Road, Bangalore - 560001 RURAL Revenue village, Habitation wise Neighbourhood Schools - 2015 Habitation Name School Code Management Lowest Highest Entry type class class class Habitation code / Ward code School Name Medium Sl.No. District : Tumkur Block : CHIKNAYAKANHALLI Revenue Village : HANDANAKERE 29180100101 29180100101 Govt. 1 8 Class 1 HANDANKERE GMHPS HANDANKERE 05 - Kannada 1 29180100101 29180100102 Govt. 1 5 Class 1 HANDANKERE GULPS HANDANKERE (URDU) 18 - Urdu 2 29180100101 29180100103 Pvt Aided 1 7 Class 1 HANDANKERE AMBEDKAR HPS HANDANKERE 05 - Kannada 3 29180100101 29180100109 Pvt Unaided 1 5 LKG HANDANKERE SRI BANASHANKARIDEVI LPS HANDNKERE 05 - Kannada 4 Revenue Village : KENGALAPURA 29180100201 29180100204 Govt. 1 5 Class 1 KENGALAPURA GLPS KENGALAPURA 05 - Kannada 5 29180100202 29180100202 Govt. 1 7 Class 1 KENGALAPURADA HATTI GHPS KENGALAPURA HATTI 05 - Kannada 6 29180100203 29180100203 Govt. 1 5 Class 1 NAYAKARA PALYA GLPS NAYAKARA PALYA 05 - Kannada 7 Revenue Village : RAMAGHATTA 29180100301 29180100301 Govt. 1 7 Class 1 RAMAGHATTA GHPS RAMGHATTA 05 - Kannada 8 29180100302 29180100302 Govt. 1 5 Class 1 TAKKALU PALYA GLPS TAKKALUPALYA 05 - Kannada 9 Revenue Village : PURADAKATTE 29180100401 29180100401 Govt. 1 5 Class 1 PURADAKATTE GLPS PURADAKATTE 05 - Kannada 10 Revenue Village : HALLITIMMALAPURA 29180100501 29180100501 Govt. 1 5 Class 1 HALLI TIMMALAPURA GLPS HALLITIMMALAPURA 05 - Kannada 11 29180100502 29180100502 Govt. 1 5 Class 1 GUNGARABAAGI GLPS GUNGABAAGI 05 - Kannada 12 Revenue Village : HUCCHANAHALLI 29180100601 29180100601 Govt. 1 5 Class 1 HUCCHANAHALLI GLPS HUCCHANAHALLI 05 - Kannada 13 e-Governance, CPI office, Bangalore 1/1/2015 -5:48:16 PM 1 Government of Karnataka O/o Commissioner for Public Instruction, Nrupatunga Road, Bangalore - 560001 RURAL Revenue village, Habitation wise Neighbourhood Schools - 2015 Habitation Name School Code Management Lowest Highest Entry type class class class Habitation code / Ward code School Name Medium Sl.No. -

Unpaid Dividend-17-18-I3 (PDF)
Note: This sheet is applicable for uploading the particulars related to the unclaimed and unpaid amount pending with company. Make sure that the details are in accordance with the information already provided in e-form IEPF-2 CIN/BCIN L72200KA1999PLC025564 Prefill Company/Bank Name MINDTREE LIMITED Date Of AGM(DD-MON-YYYY) 17-JUL-2018 Sum of unpaid and unclaimed dividend 696104.00 Sum of interest on matured debentures 0.00 Sum of matured deposit 0.00 Sum of interest on matured deposit 0.00 Sum of matured debentures 0.00 Sum of interest on application money due for refund 0.00 Sum of application money due for refund 0.00 Redemption amount of preference shares 0.00 Sales proceed for fractional shares 0.00 Validate Clear Proposed Date of Investor First Investor Middle Investor Last Father/Husband Father/Husband Father/Husband Last DP Id-Client Id- Amount Address Country State District Pin Code Folio Number Investment Type transfer to IEPF Name Name Name First Name Middle Name Name Account Number transferred (DD-MON-YYYY) 49/2 4TH CROSS 5TH BLOCK MIND00000000AZ00 Amount for unclaimed and A ANAND NA KORAMANGALA BANGALORE INDIA Karnataka 560095 54.00 23-May-2025 2539 unpaid dividend KARNATAKA 69 I FLOOR SANJEEVAPPA LAYOUT MIND00000000AZ00 Amount for unclaimed and A ANTONY FELIX NA MEG COLONY JAIBHARATH NAGAR INDIA Karnataka 560033 72.00 23-May-2025 2646 unpaid dividend BANGALORE ROOM NO 6 G 15 M L CAMP 12044700-01567454- Amount for unclaimed and A ARUNCHETTIYAR AKCHETTIYAR INDIA Maharashtra 400019 10.00 23-May-2025 MATUNGA MUMBAI MI00 unpaid -

Karnataka Golf Association
KARNATAKA GOLF ASSOCIATION LIST OF THE CANDIDATES TO BE INTERVIEWED FOR MEMBERSHIP/ASSOCIATESHIP ON TUESDAY, 20TH AUGUST 2019 SL Appl Name & Address Photo Age Occupation Membership in Golfer Proposers & Seconders No. No. & Other Clubs Dt PERMANENT MEMBER 1 2852 MR. SHANKAR SEETHARAM 55 DIRECTOR BANGALORE CLUB N MR GUPTA A,INDG031 08/3/02 #8, SANNIDHI ROAD, M/S. TRIPHASE CENTURY CLUB MR SUBBIAH K S,INDS210 BASAVANGUDI, TECHNOLOGIES (P) LTD., MR SAROHI J S,INDS027 BANGALORE-560004 MR PINTO JOHN LOUIS INDL018 MR RAJOO M J INDR012 2 2853 MR. SHYAM M GOPINATH 52 DIRECTOR CENTURY CLUB N MR KRISHNA PRASAD K,INDK079 09/3/02 #437,5TH MAIN,16TH CROSS, M/S. TRIPHASE KORAMANGALA CLUB MR RAMAPRASAD K,INDR169 SEC-6,HSR LAYOUT, TECHNOLOGIES (P) LTD., MR ANAND SRINIVASAN S,INDS271 BANGALORE-560102 MR CODANDA GANAPATHI SOMIAH (EXP) INDS237 MR BHARATH TANDON INDT033 3 2854 DR. SANDEEP B R 40 DENTAL SURGEON BANGALORE CLUB N MR NIRMAL KUMAR A R,INDN017 09/3/02 #112,LAKSHMI, 2ND MAIN ROAD, M/S. SPECIALIST DENTAL COSMOPOLITAN CLUB DR SRIRAM K R,INDS266 7TH BLOCK, JAYANAGAR , POLY CLINIC BANGALORE CITY INSTITUTE MR MARTTUKALAM M K,INDM006 BANGALORE-560070 MR NARESH KUMAR R INDK175 MR SATYA MURTHY K S INDM205 4 2856 DR. KADAPPA SHIVAPPA SATISH 60 PULMONOLOGIST VIJAYANAGAR CLUB Y MS SUSHMA G S,INDS329 11/3/02 #119, MANJULIKA , M/S. CHEST AND KSCR H/CAP MR JAYASURYA ABHIRAM,INDA118 GRUHALAXMI LAYOUT, MATERNITY CENTRE BGC 15 MR GURJAR P G,INDG004 KAMALANAGAR , MR VIJAYKANTH K C INDV063 BANGALORE-560079 MR ASHOK BABU G S (EXPIRED) INDB137 5 2858 MR. -

Starview E-Paper
2 DECCAN HERALD B Tuesday, September 15, 2009 Tomorrow: 12 pages CivicMetro 360° xxxxxxxxxxxxxx DH Realty BBMP BDA Parks and Lakes BMRDA Send your suggestions and views to [email protected] ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| Dying lakes: A continuing story ( Blue marks on the maps indicate the water bodies) The widespread outcry about Bangalore’s vanishing lakes is finally waking up the City’s civic agencies. Plans are afoot to conserve the existing water bodies and even revive some of the old ones. The agencies better speed up, because at stake is Bangalore’s very existence as a liveable City. 1973 1992 2002 2007 Interview Vanishing lakes: Time to act now Subhash Chandra NS tions, educational programmes works. Even the crores of ru- sewerage into the lakes are cit- ports say. The BU study ob- fore discharge to valleys, has way back in 1994 by the Central and government stringent pees borrowed from interna- ed by both the IISc and BBMP serves that even waste water not kept pace with water use Ground Water Board. ere are some star- norms of the State Government tional agencies to clean the lakes reports, and another study by from the apartments end up in and draining by urban popula- With no proper demarca- tling findings that and appeal from Scientific have not been fruitful,”he says. the Bangalore University. The the lakes. tion,”the BBMP report reveals. tions, the water bodies have should explain the Community and environmen- IISc report finds that of the 360 “With the availability of piped Indiscriminate drilling of been easy targets for land BHARATLAL MEENA need for urgency: talists have repeatedly failed to Forty-two lakes lost to recognised slums in the City, water supply, the importance borewells is another cause cited sharks. -
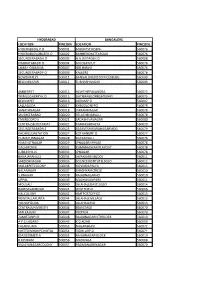
Pincodes.Pdf
HYDERABAD BANGALORE LOCATION PINCODE LOCATION PINCODE HYDERABADG.P.O 500001 MOUNTSTJOSEPH 560076 HYDERABADJUBILEEH.O 500002 BANNERGHATTAROAD 560076 SECUNDERABADH.O 500003 H.A.LIISTAGEH.O 560008 KHAIRATABADH.O 500004 MICOLAYOUT 560076 JAMA-I-OSMANIA 500007 HULIMAVU 560076 SECUNDERABADP.O 500009 KALKERE 560076 BOWENPALLY 500011 BANGALOREDISTOFFICESBLDG 560009 BEGUMBAZAR 500012 SUBHASHNAGAR 560009 AMBERPET 500013 NEWTHIPPASANDRA 560075 TRIMULGHERRYH.O 500015 GATIBANGLOREGATEWAY 560075 BEGUMPET 500016 AGRAMP.O 560007 LALLAGUDA 500017 KHBCOLONYPO 560079 SANATHNAGAR 500018 VIKRAMNAGAR 560078 MUSHEERABAD 500020 YELACHENAHALLI 560078 EMERECORD'S 500021 SADASHIVANAGAR 560080 CENTRALSECRETARIAT 500022 KAMAKSHIPALYA 560079 SECUNDERABADR.S 500025 BASAVESHWARANAGARMDG 560079 KACHIGUDASTATION 500027 KOTHANURP.O. 560077 HUMAYUNNAGAR 500028 BILEKAHALLI 560076 HIMAYATNAGAR 500029 JPNAGARIIIPHASE 560078 GACHIBOWLI 500032 KUMARASWAMYLAYOUT 560078 JUBILEEHILLS 500033 JPNAGAR 560078 BANAJARAHILLS 500034 JAYANGARIIIBLOCK 560011 SAROORNAGAR 500035 SCIENCEINSTITUTELSGSO 560012 MALAKPETCOLONY 500036 GOVINDAPALYA 560013 BALANAGAR 500037 BHASHYAMCIRCLE 560010 S.RNAGAR 500038 RAJAJINAGARHO 560010 UPPAL 500039 MADHAVANPARK 560011 MOULALI 500040 JALAHALLIEASTLSGSO 560014 RAJBHAVANROAD 500041 WHITEFIELD 560066 HALCOLONY 500042 HMTPOSTOFFICE 560013 NEWNALLAKUNTA 500044 JALAHALLIVILLAGE 560013 YOUSUFGUDA 500045 JALAHALLIHO 560013 CENTRALUNIVERSITY 500046 BSKIISTAGE 560070 MALKAJGIRI 500047 YEDIYUR 560070 ZAMISTANPUR 500048 RAJAJINAGARIVTHBLOCK 560010 A.P.S.EBOARD 500049 -

002 Adyanadka D.NO.492/2A, KEPU VILLAGE, ADYANADKA
Sl. Address No. SOL ID Branch Name Contact email id D.NO.492/2A, KEPU VILLAGE, 002 Adyanadka 9449595621 [email protected] 1 ADYANADKA Sri Krishna Upadhyaya Complex, 003 Airody 9449595625 [email protected] 2 NH66, Near Bus Stand, Sasthana Plot No. 1185, First Floor, Srinivas 005 Almel Nilaya, Indi Road, Near APMC, 9449595573 [email protected] 3 Almel TAPOVANA COMPLEX, SHIRAL 006 Anavatti KOPPA - HANGAL ROAD, 9449595401 [email protected] 4 ANAVATTI Ground Floor, Bharath Complex, 007 Arehalli 9449595402 [email protected] 5 Belur Road, Arehalli 6 009 Arsikere-Main LAKSHMI, B.H.ROAD, ARSIKERE 9449595404 [email protected] “Ganesh Ram Arcade”, No.213, B 010 Ayanur 9449595520 [email protected] 7 H Road, Ayanur Ist FLOOR, LOURDES COMPLEX, 011 Amtady AMTADY, LORETTO POST, 9449595624 [email protected] 8 AMTADY, BANTWAL TALUK. RAMAKRISHNA NILAYA, POST 012 Aikala 9449595622 [email protected] 9 KINNIGOLI, AIKALA Door No. 1/89(11), SY. No. 78/12, Old SY No. 78/4P2, “Sinchana Complex”, Ground Floor, 013 Amasebail 9449595626 [email protected] Amasebail Siddapura Road, Amasebail Village, Kundapura 10 Taluk, Udupi District – 576227 OPP.PUSHPANJALI TALKIES, 014 Agali 8500801827 [email protected] 11 MADAKASIRA ROAD, AGALI. GROUND FLOOR, NO.47/1, SRI 015 Aladangady LAXMI NILAYA, MAIN ROAD, ANE 9449595623 [email protected] 12 MAHAL, ALADANGADY Ist FLOOR, DURGA Udupi-Adi 016 INTERNATIONAL BUILDING, 9449595595 [email protected] Udupi 13 UDUPI-MALPE ROAD, UDUPI BUILDING1(817), OPP.HOTEL Goa-Alto 017 O'COQUEIRO, PANAJI-MAPUSA 9423057235 [email protected] Porvorim 14 HIGH WAY, ALTO PORVORIM SUJATHA COMPLEX, MANIPAL Udupi- 018 CROSS ROAD, AMBAGILU- 9448463283 [email protected] Ambagilu 15 UDUPI CTS No. -

Pel2011-12 Iepf 2
Note: This sheet is applicable for uploading the particulars related to the unclaimed and unpaid amount pending with company. Make sure that the details are in accordance with the information already provided in e-form IEPF-2 Date Of AGM(DD-MON-YYYY) CIN/BCIN L07010KA1997PLC022322 Prefill Company/Bank Name PRESTIGE ESTATES PROJECTS LIMITED 23-Sep-2016 Sum of unpaid and unclaimed dividend 32538.00 Sum of interest on matured debentures 0.00 Sum of matured deposit 0.00 Sum of interest on matured deposit 0.00 Sum of matured debentures 0.00 Sum of interest on application money due for refund 0.00 Sum of application money due for refund 0.00 Redemption amount of preference shares 0.00 Sales proceed for fractional shares 0.00 Validate Clear Proposed Date of Investor First Investor Middle Investor Last Father/Husband Father/Husband Father/Husband Last DP Id-Client Id- Amount Address Country State District Pin Code Folio Number Investment Type transfer to IEPF Name Name Name First Name Middle Name Name Account Number transferred (DD-MON-YYYY) BALASUBRAMA AISWARYA HOUSE PRESIN30023912279 Amount for unclaimed and A NIAN NA CHETHIMATTOM PALA P O INDIA Kerala 686575 022 unpaid dividend 10.80 29-Sep-2017 SITE NO 25 KUVEMPUNAGAR BEHIND PUSHPANJALI TALKIES CHITRADURGA HIRIYUR PRESIN30192630862 Amount for unclaimed and A M ASTHEER ANEEFARAWTHER KARNATAKA INDIA Karnataka 572143 632 unpaid dividend 72.00 29-Sep-2017 PUNDALEEKARA S/O RAMALINGARAO OLD BUS PRES1203840000825 Amount for unclaimed and A R O NA STAND ROAD SIRA INDIA Karnataka 572137 294 unpaid dividend 246.00 29-Sep-2017 ABDULRAHIMA NEAR URBAN CO-OP BANK PRESIN30113526242 Amount for unclaimed and N KITTUR NA LAXMESHWAR TAL SHIRAHATTI INDIA Karnataka 582116 585 unpaid dividend 24.00 29-Sep-2017 C-142, B. -

Metro Cities Atm List City Address
METRO CITIES ATM LIST CITY ADDRESS BANGALORE AEGIS LIMITED RELIANCE JIO PVT LTD C/O MIND COMP TECH PARK ROAD NO 7 EPIP AREA WHITEFIELD BEHIND L&T INFOTECH BENGALURU KT IN 560066 FINANCE AND BUSINESS OPERATIONS TEAM INCTURE TECHNOLOGIES PVT LTD 3RD FLOOR, BLOCK A, SALARPURIA AURA KADUBEESANAHALLI OUTER RING ROAD BANGALORE ‘SILVER PALMS’, #3, PALMGROVE ROAD, VICTORIA LAYOUT, BENGALURU - 560047 NO.73/1-1, GROUND FLOOR, KRISHNA INFANTRY ROAD, BENGALURU-560001 AXIS BANK LTD MAIN BRANCH NO 9 M G ROAD BLOCK A BANGALORE 560 001 AXIS BANK LTD MAIN BRANCH NO 9 M G ROAD BLOCK A BANGALORE 560 001 AXIS BANK ATM VALTECH 30/A GROUND FLOOR J P NAGAR SARAKKI 3RD PHASE 1ST MAIN ROAD 3RD STAGE INDUSTRIAL SUBURB BANGALORE AXIS BANK ATM ANAGHA NO4 DEVASANDRA NEW BEL RD NEXT TO COFFEE DAY ANJANYA TEMPLE STREET BANGALORE 560012 AXIS BANK ATM ANAGHA NO4 DEVASANDRA NEW BEL RD NEXT TO COFFEE DAY ANJANYA TEMPLE STREET BANGALORE 560012 AXIS BANK ATM ASC CENTER & COLLEGE REGIMENTAL SHOPPING COMPLEX ( SOUTH) ASC CENTER & COLLEGE AGRAM BANGALORE 560 007 AXIS BANK ATM ASC CENTER & COLLEGE REGIMENTAL SHOPPING COMPLEX ASC CENTER & COLLEGE AGRAM BANGALORE 560 007 AXIS BANK ATM ASC CENTER & COLLEGE REGIMENTAL SHOPPING COMPLEX ASC CENTER & COLLEGE AGRAM BANGALORE 560 007 AXIS BANK ATM SENA POLICE CORPS KENDRA AUR SCHOOL (CMP CENTER & SCHOOL) BANGALORE 560025 AXIS BANK ATM TATA ELEXSI LTD ITPL ROAD WHITEFIELD BANGALORE 560 048 AXIS BANK ATM GOLFLINKS SOFTWARE PARK 24/7 CUSTOMER GOLFLINKS SOFTWARE PARK PVT LTD 2/13 AND 5/1 CHALLAGHATTA VILLAGE VARTHUR HOBLI BANGALORE 560 071 -

Sub Centre List As Per HMIS SR
Sub Centre list as per HMIS SR. DISTRICT NAME SUB DISTRICT FACILITY NAME NO. 1 Bagalkote Badami ADAGAL 2 Bagalkote Badami AGASANAKOPPA 3 Bagalkote Badami ANAVALA 4 Bagalkote Badami BELUR 5 Bagalkote Badami CHOLACHAGUDDA 6 Bagalkote Badami GOVANAKOPPA 7 Bagalkote Badami HALADURA 8 Bagalkote Badami HALAKURKI 9 Bagalkote Badami HALIGERI 10 Bagalkote Badami HANAPUR SP 11 Bagalkote Badami HANGARAGI 12 Bagalkote Badami HANSANUR 13 Bagalkote Badami HEBBALLI 14 Bagalkote Badami HOOLAGERI 15 Bagalkote Badami HOSAKOTI 16 Bagalkote Badami HOSUR 17 Bagalkote Badami JALAGERI 18 Bagalkote Badami JALIHALA 19 Bagalkote Badami KAGALGOMBA 20 Bagalkote Badami KAKNUR 21 Bagalkote Badami KARADIGUDDA 22 Bagalkote Badami KATAGERI 23 Bagalkote Badami KATARAKI 24 Bagalkote Badami KELAVADI 25 Bagalkote Badami KERUR-A 26 Bagalkote Badami KERUR-B 27 Bagalkote Badami KOTIKAL 28 Bagalkote Badami KULAGERICROSS 29 Bagalkote Badami KUTAKANAKERI 30 Bagalkote Badami LAYADAGUNDI 31 Bagalkote Badami MAMATGERI 32 Bagalkote Badami MUSTIGERI 33 Bagalkote Badami MUTTALAGERI 34 Bagalkote Badami NANDIKESHWAR 35 Bagalkote Badami NARASAPURA 36 Bagalkote Badami NILAGUND 37 Bagalkote Badami NIRALAKERI 38 Bagalkote Badami PATTADKALL - A 39 Bagalkote Badami PATTADKALL - B 40 Bagalkote Badami SHIRABADAGI 41 Bagalkote Badami SULLA 42 Bagalkote Badami TOGUNSHI 43 Bagalkote Badami YANDIGERI 44 Bagalkote Badami YANKANCHI 45 Bagalkote Badami YARGOPPA SB 46 Bagalkote Bagalkot BENAKATTI 47 Bagalkote Bagalkot BENNUR Sub Centre list as per HMIS SR. DISTRICT NAME SUB DISTRICT FACILITY NAME NO. -
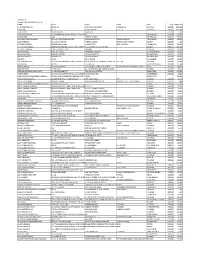
Kopran Ltd Unpaid Dividend 2020-21 Interim NAME ADD1 ADD2 ADD3 CITY PIN AMOUNT A
Kopran Ltd Unpaid dividend 2020-21 Interim NAME ADD1 ADD2 ADD3 CITY PIN AMOUNT A . PADMANABHAN NO.24/16 VEDACHALA GARDEN MANDAVELI CHENNAI 600028 2700.00 A KUMAR 24 1 1 ALIPORE ROAD FLAT 12 CALCUTTA 700027 1500.00 A MUTHIAH 496 KK NAGAR MADURAI 625020 9.00 A N NAGARAJAIAH C/O DHONDUSA EXPORT HOUSE 273 S C ROAD BANGALORE 560009 150.00 A NAGAVENI 8-82 TEMPLE ALWAL HYDERABAD 500010 798.00 A S G PAVOON THILAGAM OLD NO 133/2G NEW NO 452 KAMARAJAR ROAD VIRUDHUNAGAR TAMILNADU 626123 450.00 A SADIGBASHA 9/1/124 WARD 8/9 INDHRA NAGAR VEERAKKALPUTHUR SP SALEM 636403 1.50 A SUNDARARAJAN NO 238 MELATHERU KAKKARAI PO ORATHANADU THANJAVUR 614625 225.00 A T FRANCIS XAVIER AMBALAMKANDAM HOUSE, THOTTAKOM P O VAIKOM, KOTTAYAM DIST KERELA 686145 225.00 A. ABDUL SUBHAN 13/B C V MOSES STREET PAMMAL CHENNAI 600075 150.00 A. RAMREDDY H NO 6-1-118/3, PADMARAO NAGAR, SECUNDARABAD 500025 225.00 A.K.SRIVASTAVA KOPRAN LIMITED, VILLAGE-SAVROLI, RAIGAD(DIST) 410202 150.00 A.K.SRIWASTAVA KOPRAN LTD., VILLAGE SAVROLI, RAIGAD(DIST) 410202 225.00 A.MAKIN 23/25 BUXI BAZAR ALLAHABAD 211003 150.00 A.P SUBRAMANIAN 14 MULUND POONAM CO OP HSG SOCIETY IIDR FL R.P ROAD B/H MUNCIPAL HOSPITAL MULUND MUMBAI 400080 150.00 A.S.KUMAR B-144A, SECTOR-26, GHAZIABAD(DIST) 201301 225.00 AABHA JANGRA HOUSE NUMBER-1 GOVT POLYTECHNIC BOYS SIRSA NEAR GOVT POLYTECHNIC SCHOOL SIRSA 125055 300.00 AASHISH VYAS 5, PRAGATI, 60, J K MEHTA ROAD, SANTACRUZ(W) BOMBAY 400054 75.00 AASHITA ASHWINKUMAR PATEL 23-NANDANVAN SOC B/H RAILWAY STATON VADODARA 390005 300.00 AASIF U SURTI C/O HANI INDUSTRIES LTD 211-212 SAMPANNANAVARANGPURA AHMEDABAD 380009 75.00 ABBAS FAKHRUDDIN FURNITUREWALA PO BOX 6218 C/O ELEMENT MIDDLE EAST DUBAI DUBAI UAE 237.00 ABDUL MUJEER KHAN 22/109,NEAR HALL TALIM MASQUE POTTERS STREET A.P.