Silvical Characteristics of Chinkapin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recommended Trees for Winnetka
RECOMMENDED TREES FOR WINNETKA SHADE TREES Common_Name Scientific_Name Ohio Buckeye Acer galbra Miyabe Maple Acer miyabei Black Maple Acer nigrum Norway Maple Acer plantanoides v. ___ Sugar Maple (many cultivars) Acer saccharum Shangtung Maple Acer truncatum Autumn Blaze or Marmo Maple Acer x freemanii Red Horsechestnut Aesculus x carnea 'Briotii' Horsechestnut Aesulus hippocastanum Alder Alnus glutinosa Yellowwood Caldrastis lutea Upright European Hornbeam Carpinus betulus “Fastigata” American Hornbeam Carpinus carolinians Hickory Carya ovata Catalpa Catalpa speciosa Hackberry Celtis occidentalis Katsuratree Cercidiphyllum japonicum Turkish Filbert Corylus colurna American Beech Fagus grandifolia Green Beech Fagus sylvatica European Beech Fagus sylvatica Ginkgo Ginkgo biloba Thornless Honeylocust Gleditsia triacanthos inermis Kentucky Coffeetree Gymnocladus dioica Goldenraintree Koelreuteria paniculata Sweetgum Liquidambar styraciflua Tulip Tree Liriodendron tulipfera Black gum, Tupelo Liriodendron tulipfera Hophornbeam Ostrya virginiana Corktree Phellodendron amurense Exclamation Plantree Plantanus x aceerifolia Quaking Aspen Populus tremuloides Swamp White Oak Quercus bicolor Shingle Oak Quercus imbricaria Bur Oak Quercus macrocarpa Chinkapin Oak Quercus muehlenbergii English Oak Quercus robur Red Oak Quercus rubra Schumard Oak Quercus shumardii Black Oak Quercus velutina May 2015 SHADE TREES Common_Name Scientific_Name Sassafras Sassafras albidum American Linden Tilia Americana Littleleaf Linden (many cultivars) Tilia cordata Silver -

GIS Handbook Appendices
Aerial Survey GIS Handbook Appendix D Revised 11/19/2007 Appendix D Cooperating Agency Codes The following table lists the aerial survey cooperating agencies and codes to be used in the agency1, agency2, agency3 fields of the flown/not flown coverages. The contents of this list is available in digital form (.dbf) at the following website: http://www.fs.fed.us/foresthealth/publications/id/id_guidelines.html 28 Aerial Survey GIS Handbook Appendix D Revised 11/19/2007 Code Agency Name AFC Alabama Forestry Commission ADNR Alaska Department of Natural Resources AZFH Arizona Forest Health Program, University of Arizona AZS Arizona State Land Department ARFC Arkansas Forestry Commission CDF California Department of Forestry CSFS Colorado State Forest Service CTAES Connecticut Agricultural Experiment Station DEDA Delaware Department of Agriculture FDOF Florida Division of Forestry FTA Fort Apache Indian Reservation GFC Georgia Forestry Commission HOA Hopi Indian Reservation IDL Idaho Department of Lands INDNR Indiana Department of Natural Resources IADNR Iowa Department of Natural Resources KDF Kentucky Division of Forestry LDAF Louisiana Department of Agriculture and Forestry MEFS Maine Forest Service MDDA Maryland Department of Agriculture MADCR Massachusetts Department of Conservation and Recreation MIDNR Michigan Department of Natural Resources MNDNR Minnesota Department of Natural Resources MFC Mississippi Forestry Commission MODC Missouri Department of Conservation NAO Navajo Area Indian Reservation NDCNR Nevada Department of Conservation -

Native Nebraska Woody Plants
THE NEBRASKA STATEWIDE ARBORETUM PRESENTS NATIVE NEBRASKA WOODY PLANTS Trees (Genus/Species – Common Name) 62. Atriplex canescens - four-wing saltbrush 1. Acer glabrum - Rocky Mountain maple 63. Atriplex nuttallii - moundscale 2. Acer negundo - boxelder maple 64. Ceanothus americanus - New Jersey tea 3. Acer saccharinum - silver maple 65. Ceanothus herbaceous - inland ceanothus 4. Aesculus glabra - Ohio buckeye 66. Cephalanthus occidentalis - buttonbush 5. Asimina triloba - pawpaw 67. Cercocarpus montanus - mountain mahogany 6. Betula occidentalis - water birch 68. Chrysothamnus nauseosus - rabbitbrush 7. Betula papyrifera - paper birch 69. Chrysothamnus parryi - parry rabbitbrush 8. Carya cordiformis - bitternut hickory 70. Cornus amomum - silky (pale) dogwood 9. Carya ovata - shagbark hickory 71. Cornus drummondii - roughleaf dogwood 10. Celtis occidentalis - hackberry 72. Cornus racemosa - gray dogwood 11. Cercis canadensis - eastern redbud 73. Cornus sericea - red-stem (redosier) dogwood 12. Crataegus mollis - downy hawthorn 74. Corylus americana - American hazelnut 13. Crataegus succulenta - succulent hawthorn 75. Euonymus atropurpureus - eastern wahoo 14. Fraxinus americana - white ash 76. Juniperus communis - common juniper 15. Fraxinus pennsylvanica - green ash 77. Juniperus horizontalis - creeping juniper 16. Gleditsia triacanthos - honeylocust 78. Mahonia repens - creeping mahonia 17. Gymnocladus dioicus - Kentucky coffeetree 79. Physocarpus opulifolius - ninebark 18. Juglans nigra - black walnut 80. Prunus besseyi - western sandcherry 19. Juniperus scopulorum - Rocky Mountain juniper 81. Rhamnus lanceolata - lanceleaf buckthorn 20. Juniperus virginiana - eastern redcedar 82. Rhus aromatica - fragrant sumac 21. Malus ioensis - wild crabapple 83. Rhus copallina - flameleaf (shining) sumac 22. Morus rubra - red mulberry 84. Rhus glabra - smooth sumac 23. Ostrya virginiana - hophornbeam (ironwood) 85. Rhus trilobata - skunkbush sumac 24. Pinus flexilis - limber pine 86. Ribes americanum - wild black currant 25. -

Common Name (S) Genus Species TREE SPECIES NATIVE to INDIANA with Common and Scientific Names
TREE SPECIES NATIVE TO INDIANA with common and scientific names Common name (s) Genus Species Ash Black Fraxinus nigra Blue Fraxinus quadrangulata Green (Red) Fraxinus pennsylvanica Pumpkin Fraxinus profunda White Fraxinus americana Aspen Big-tooth Populus grandidentata Quaking Populus tremuloides Basswood, American Tilia americana Beech, American Fagus grandifolia Birch Gray Betula populifolia Paper Betula papyrifera River Betula nigra Yellow Betula alleghaniensis Black gum (sour, tupelo) Nyssa sylvatica Buckeye Ohio Aesculus glabra Yellow Aesculus aiton Butternut Juglans cinerea Catalpa, northern Catalpa speciosa Cedar Eastern red Juniperus virginiana Northern white Thuja occidentalis Cherry Black Prunus serotina Chestnut, American Castanea dentata Coffeetree, Kentucky Gymnocladus dioica Cottonwood Eastern Populus deltoides Swamp Populus heterophylla Crabapple Sweet, American Pyrus coronaria Prairie Pyrus ioensis Cypress, bald Taxiodium distichum Dogwood, flowering Cornus florida Elm American (White) Ulmus americana Rock Ulmus thomasii Slippery (Red) Ulmus rubra Winged Ulmus alata Hackberry Southern hackberry (Sugar) Celtis laevigata Northern hackberry Celtis occidentalis Hawthorn TREE SPECIES NATIVE TO INDIANA with common and scientific names Common name (s) Genus Species Cockspur-thorn Crataegus crus-galli Dotted Crataegus punctata Downy Crataegus mollis Green Crataegus viridis Hemlock, eastern Tsuga canadensis Hickory Bitternut Carya cordiformis Mockernut Carya tomentosa Pale (Sand) Carya pallida Pignut Carya glabra Red Carya ovalis -

Climate-Ready Tree List
Location Type 1 - Small Green Stormwater Infrastructure (GSI) Features Location Characteristics Follows “Right Tree in the Right Place” Low Points Collect Stormwater Runoff Soil Decompacted to a Depth ≥ 18” May Have Tree Trenches, Curb Cuts, or Scuppers Similar Restrictions to Location Type 5 Examples:Anthea Building, SSCAFCA, and South 2nd St. Tree Characteristics Recommended Trees Mature Tree Height: Site Specific Celtis reticulata Netleaf Hackberry Inundation Compatible up to 96 Hours. Cercis canadensis var. mexicana* Mexican Redbud* Cercis occidentalis* Western Redbud* Pollution Tolerant Cercis reniformis* Oklahoma Redbud* Cercis canadensis var. texensis* Texas Redbud* Crataegus ambigua* Russian Hawthorne* Forestiera neomexicana New Mexico Privet Fraxinus cuspidata* Fragrant Ash* Lagerstroemia indica* Crape Myrtle* Pistacia chinensis Chines Pistache Prosopis glandulosa* Honey Mesquite* Prosopis pubescens* Screwbean Mesquite* Salix gooddingii Gooding’s Willow Sapindus saponaria var. drummondii* Western Soapberry* * These species have further site specific needs found in Master List Photo Credit: Land andWater Summit ClimateReady Trees - Guidelines for Tree Species Selection in Albuquerque’s Metro Area 26 Location Type 2 - Large Green Stormwater Infrastructure (GSI) Features Location Characteristics Follows “Right Tree in the Right Place” Low Points Collect Stormwater Runoff Soil Decompacted to a Depth ≥ 18” May Have Basins, Swales, or Infiltration Trenches Examples: SSCAFCA landscaping, Pete Domenici Courthouse, and Smith Brasher Hall -

Introduction to the Southern Blue Ridge Ecoregional Conservation Plan
SOUTHERN BLUE RIDGE ECOREGIONAL CONSERVATION PLAN Summary and Implementation Document March 2000 THE NATURE CONSERVANCY and the SOUTHERN APPALACHIAN FOREST COALITION Southern Blue Ridge Ecoregional Conservation Plan Summary and Implementation Document Citation: The Nature Conservancy and Southern Appalachian Forest Coalition. 2000. Southern Blue Ridge Ecoregional Conservation Plan: Summary and Implementation Document. The Nature Conservancy: Durham, North Carolina. This document was produced in partnership by the following three conservation organizations: The Nature Conservancy is a nonprofit conservation organization with the mission to preserve plants, animals and natural communities that represent the diversity of life on Earth by protecting the lands and waters they need to survive. The Southern Appalachian Forest Coalition is a nonprofit organization that works to preserve, protect, and pass on the irreplaceable heritage of the region’s National Forests and mountain landscapes. The Association for Biodiversity Information is an organization dedicated to providing information for protecting the diversity of life on Earth. ABI is an independent nonprofit organization created in collaboration with the Network of Natural Heritage Programs and Conservation Data Centers and The Nature Conservancy, and is a leading source of reliable information on species and ecosystems for use in conservation and land use planning. Photocredits: Robert D. Sutter, The Nature Conservancy EXECUTIVE SUMMARY This first iteration of an ecoregional plan for the Southern Blue Ridge is a compendium of hypotheses on how to conserve species nearest extinction, rare and common natural communities and the rich and diverse biodiversity in the ecoregion. The plan identifies a portfolio of sites that is a vision for conservation action, enabling practitioners to set priorities among sites and develop site-specific and multi-site conservation strategies. -
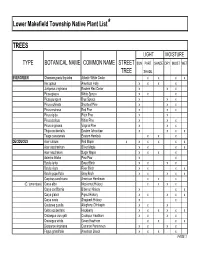
Native Plant List Trees.XLS
Lower Makefield Township Native Plant List* TREES LIGHT MOISTURE TYPE BOTANICAL NAME COMMON NAME STREET SUN PART SHADE DRY MOIST WET TREE SHADE EVERGREEN Chamaecyparis thyoides Atlantic White Cedar x x x x IIex opaca American Holly x x x x Juniperus virginiana Eastern Red Cedar x x x Picea glauca White Spruce x x x Picea pungens Blue Spruce x x x Pinus echinata Shortleaf Pine x x x Pinus resinosa Red Pine x x x Pinus rigida Pitch Pine x x Pinus strobus White Pine x x x Pinus virginiana Virginia Pine x x x Thuja occidentalis Eastern Arborvitae x x x x Tsuga canadensis Eastern Hemlock xx x DECIDUOUS Acer rubrum Red Maple x x x x x x Acer saccharinum Silver Maple x x x x Acer saccharum Sugar Maple x x x x Asimina triloba Paw-Paw x x Betula lenta Sweet Birch x x x x Betula nigra River Birch x x x x Betula populifolia Gray Birch x x x x x Carpinus caroliniana American Hornbeam x x x (C. tomentosa) Carya alba Mockernut Hickory x x x x Carya cordiformis Bitternut Hickory x x x Carya glabra Pignut Hickory x x x x x Carya ovata Shagbark Hickory x x Castanea pumila Allegheny Chinkapin xx x Celtis occidentalis Hackberry x x x x x x Crataegus crus-galli Cockspur Hawthorn x x x x Crataegus viridis Green Hawthorn x x x x Diospyros virginiana Common Persimmon x x x x Fagus grandifolia American Beech x x x x PAGE 1 Exhibit 1 TREES (cont'd) LIGHT MOISTURE TYPE BOTANICAL NAME COMMON NAME STREET SUN PART SHADE DRY MOIST WET TREE SHADE DECIDUOUS (cont'd) Fraxinus americana White Ash x x x x Fraxinus pennsylvanica Green Ash x x x x x Gleditsia triacanthos v. -

Exhibit L1 – List of Native Overstory Trees in Frederick County *Aste
List of Native Overstory Trees In Middletown, MD Source: Exhibit L1 – List of Native Overstory Trees in Frederick County *Asterisks indicate additions to List made because of naturalization or other reasons, assessed by Frederick County Planning Department Conifers - Scientific Name Common Name(s) (and Broad-leafed Evergreens) Juniperus virginiana (Eastern) Red Cedar Pinus echinata Shortleaf Pine Pinus punguns Table Mountain Pine Pinus rigida Pitch Pine Pinus strobus White Pine Pinus virginiana Virginia Pine *Taxodium distichum *Bald Cypress Tsuga canadensis Eastern Hemlock Hardwoods – Scientific Name Common Name(s) Acer negundo Box-Elder Acer nigrum Black Maple Acer rubrum Red Maple Acer saccharinum Silver Maple (Invasive Native – not recommended) Acer saccharum Sugar Maple Alnus rugosa, (serrulata) Smooth Alder (Hazel Alder) Betula lenta Black Birch (Sweet Birch) Betula nigra River Birch (Black Birch) Carpinus caroliniana Blue Beech (American Hornbeam, Musclewood, Ironwood) Carya cordiformis Bitternut Hickory Carya glabra Pignut Hickory Carya ovata Shagbark Hickory Carya pallida Sand Hickory Carya tomentosa Mockernut Hickory Castanea dentata (American) Chestnut Castanea pumila Eastern or Allegheny Chinkapin Celtis occidentalis Hackberry Fagus grandifolia (American) Beech Fraxinus species–There is a temporary, albeit indefinite ban on Ash trees in Frederick County. Juglans cinerea Butternut Juglans nigra Black Walnut *Liquidambar styraciflua *Sweet Gum Liriodendron tulipifera Tulip Tree (Tulip Poplar) Magnolia acuminata Cucumber Tree -

A NATURAL HERITAGE INVENTORY of MIFFLIN COUNTY, PENNSYLVANIA June 2007
A NATURAL HERITAGE INVENTORY OF MIFFLIN COUNTY, PENNSYLVANIA June 2007 Prepared by: Pennsylvania Natural Heritage Program Western Pennsylvania Conservancy 208 Airport Drive Middletown, Pennsylvania 17057 Submitted to: Mifflin County Planning Commission 20 North Wayne Street Lewistown, PA 17044 This project was funded in part by a state grant from the Department of Conservation and Natural Resources Wild Resource Conservation Program. Additional support was provided by the Department of Community & Economic Development. Additional funding was provided by the U.S. Fish and Wildlife Service through State Wildlife Grants program grant T-2, administered through the Pennsylvania Game Commission and the Pennsylvania Fish and Boat Commission. ii A Natural Heritage Inventory of Mifflin County, Pennsylvania 2007 Prepared by: Pennsylvania Natural Heritage Program (PNHP) Western Pennsylvania Conservancy (WPC) 208 Airport Drive Middletown, PA 17057 Donna Bowers, Administration Lucy Boyce, Seasonal Field Ecologist Anthony F. Davis, Senior Ecologist Jeremy Deeds, Aquatic Zoology Coordinator Alice Doolittle, Conservation Assistant Charlie Eichelberger, Herpetologist Kathy Derge Gipe, Herpetologist William (Rocky) Gleason, County Inventory Coordinator Jim Hart, Mammalogist Rita Hawrot, Terrestrial Zoology Coordinator Denise Johnson, Assistant County Inventory Ecologist Susan Klugman, Conservation Information Manager John Kunsman, Senior Botanist Betsy Ray Leppo, Invertebrate Zoologist Trina Morris, County Inventory Ecologist Betsy Nightingale, Aquatic -

Hemileuca Lucina Henry Edwards, H. Nevadensis Stretch, Anisota Senatoria 0
Journal of the Lepidopterists' Society 38(1). 1984. 51-56 TWO INTERESTING ARTIFICIAL HYBRID CROSSES IN THE GENERA HEMILEUCA AND ANISOTA (SATURNIIDAE) RICHARD STEVEN PEIGLER1 303 Shannon Drive, Greenville, South Carolina 29615 AND BENJAMIN D. WILLIAMS The Lawrence Academy, Groton, Massachusetts 01450 ABSTRACT. Two crosses were reared to the adult stage with saturniid moths from different areas of the United States. These were Hemileuca lucina 5 x H. nevadensis Q reared in Massachusetts and Texas on Salix, and Anisata senataria 5 x A. aslari Q reared in Connecticut on Quercus caccinea. Larvae and adults of both crosses were interme diate. Descriptions and figures of the hybrids are given. Several isolating mechanisms between the parent species were tested and are discussed. Dozens of artificial crosses in the Saturniidae have been successfully reared since the previous century, but virtually all of these have in volved species of the subfamily Saturniinae. This paper deals with two remarkable crosses obtained by the junior author utilizing small satur niid moths belonging to the subfamilies Hemileucinae and Ceratocam pinae.2 In both crosses, species native to the Southwest were reared in the Northeast and females from those rearings attracted congeneric diurnal males native to the Northeast. The species involved were Hemileuca lucina Henry Edwards, H. nevadensis Stretch, Anisota senatoria 0. E. Smith) and A. oslari W. Rothschild. For information on the adult morphology, wing pattern, immature stages, hostplants, reproductive behavior, and geographical distributions of these four parent species, the reader is referred to works by Ferguson (1971) and Riotte and Peigler (1981). Hemileuca lucina <3 x H. -

Deerwood Arboretum
Species No. Common Name Scientific Name Species No. Common Name Scientific Name Tree Identification 1 sweetbay magnolia Magnolia virginiana 35 buckthorn bumelia Bumelia lycioides Trail Guide 2 sycamore Plantanus accidentalis 36 carolina silverbell Halesia tetraptera 3 white ash Fraxinus Americana 37 sourwood Oxydendrum arboretum 4 American sweetgum Liquidambar styraciflua 38 American yellowwood Cladrastis kentukea Trees are among the largest and 5 sugar maple Acer saccharum 39 carolina buckthorn Rhamnus caroliniana most useful plants on earth. 6 flowering dogwood Cornus florida 40 tree of heaven Ailanthus altissima 7 common bladcypress Taxodium distichum 41 black willow Salix nigra Collectively, trees comprise 8 red maple Acer rubrum 42 Eastern cottonwood Populous deltoids forests that provide food, 9 Eastern redbud Cercis Canadensis 43 thornless common honeylocust Gleditsia triacanthos var.inermis shelter and innumerable 10 tulip poplar Liriodendron tulipifera 44 willow oak Quercus phellos benefits for native wildlife and 11 common hackberry Celtis occidentalis 45 American elm Ulmus Americana humans alike. From the Smokey 12 bitternut hickory Carya cordiformis 46 swamp dogwood Cornus foemina Mountains in East Tennessee to 13 Eastern white pine Pinus strobus 47 common pawpaw Asimina triloba the Mississippi River in the 14 river birch Betula nigra 48 shellbark hickory Carya laciniosa West, few states have as many 15 black cherry Prunus serotine 49 No assigned species Prunus serotine tree species as Tennessee. 16 Eastern hemlock Tsuga Canadensis -

Chinquapin Oak Propagation Protocol
Protocol Information USDA NRCS - Appalachian Plant Materials Center P. O. Box 390 Alderson, West Virginia 24910 304-445-3005 304-445-7049 [email protected] http://plant-materials.nrcs.usda.gov/wvpmc Family Scientific Name: Fagaceae Family Common Name: Beech Scientific Name: Quercus muehlenbergii Engelm. Common Name: chinkapin oak Species Code: QUMU Ecotype: Stones River source General Distribution: Chinquapin oak is common throughout the eastern two-thirds of the continental United States with the exception of the New England states of New Hampshire, and Maine and the North Central states of North Dakota and South Dakota. Known Invasiveness: None Propagation Goal: Plants Propagation Method: Seed Product Type: Bareroot (field grown) Stock Type: 1-0 Time To Grow: 2 Years Target Specifications: A second spring seedling ranging in height from 8" to 16" with a 1/16" to 3/16" caliper stem and a compact, well developed tap root system. Propagule Collection: Seeds are collected from established natural stands within the confines of Stones River National Battlefield in the fall immediately after the acorns have matured and begun to fall from the tree. Propagule Processing: Chinquapin oak reproduces readily from seed. Seed has no physiological dormancy and should be sown immediately upon harvest for best results. Fall sown seed typically exhibits >90% germination, while seeds stored overwinter exhibit greatly reduced germination; typically <50%. Pre-Planting Treatments: Seed may be floated in water to help determine viability. Seed that floats is normally poorly filled and has low or no viability. Floaters are discarded, while the seed that sinks is retained for planting.