Report Ongoing Collapse of Coral-Reef Shark Populations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

White-Tip Reef Shark (Triaenodon Obesus) Michelle S
White-tip Reef Shark (Triaenodon obesus) Michelle S. Tishler Common Name There are several common names for the Triaenodon obesus, which usually describes the “white tips” on their dorsal and caudal fins. Common names include: White-tip Reef Shark, Blunthead Shark, Light-Tip Shark and Reef Whitetip. Names in Spanish Cazón, Cazón Coralero Trompacorta and Tintorera Punta Aleta Blanca. Taxonomy Domain Eukarya Kingdom Anamalia Phylum Chordata Class Chondrichthyes Order Carcharhiniformes Family Carcharhinidae Genus Triaenodon Species obesus Nearest relatives Sharks are cartilaginous fishes in the class Chondrichthyes with skates, rays and other sharks. Within the family Carcharhinidae (requiem sharks), the White-tip Reef Shark is related to the Galapagos Shark, Bull Shark, Oceanic Whitetip, Tiger Shark and Blue Sharks. The White-tip Reef Shark does not share their genus name with any other organism. Island They are found amongst the reefs surrounding most or all of the Galapagos Islands. Geographic range White-tip Sharks range geographically from Costa Rica, Ecuador, Galapagos, Cocos, South Africa, Red Sea, Pakistan and etc. to primarily residing in the Indo-West Pacific region. (Red region indicates distribution of White-tip Reef Shark) Habitat Description As described in their name, White-tip Reef Sharks live amongst coral reefs with a home range of a couple square miles. They are also found in sandy patches and deeper waters. During the day these sharks tend to rest on the seabed or within caves and crevices. Physical description White-tip Reef sharks are named after the white tip on the dorsal (first and sometimes second) fins, and caudal fin lobes. -

Shark Populations in Marine Protected Areas in Raja Ampat
Running Head: SHARK POPULATIONS IN MARINE PROTECTED AREAS IN RAJA AMPAT DIVERSITY AND ABUNDANCE OF SHARKS IN NONO----TAKETAKE AND FISHED SITES IN THE MARINE PROTECTED AREA NETWORK OF RAJA AMPAT, WEST PAPUA, INDONESIA, USING BAITED REMOTE UNDERWATER VIDEO (BRUVs(BRUVs)))) By ANGELA JUNE ELIZE BEER B.Sc., Queen’s University, 2001 B.Ed., Lakehead University, 2003 A thesis submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in ENVIRONMENT AND MANAGEMENT We accept this thesis as conforming to the required standard .......................................................... Dr. Steven Lindfield, Thesis Supervisor University of Western Australia .......................................................... Dr. Audrey Dallimore, Thesis Coordinator School of Environment and Sustainability .......................................................... Dr. Chris Ling, Director School of Environment and Sustainability ROYAL ROADS UNIVERSITY February 2015 © Angela June Elize Beer, 2015 SHARK POPULATIONS IN MARINE PROTECTED AREAS IN RAJA AMPAT 2 Abstract Sharks are essential elements for healthy marine ecosystems and require conservation. A new law has been passed for their protection in Raja Ampat, Indonesia, yet there is a lack of knowledge about their current status in the region. This research quantifies diversity and abundance of shark populations in two areas (Penemu and Dampier Straight) within Raja Ampat, and provides a comprehensive baseline for the areas to evaluate management strategies. Baited remote underwater video systems were used to survey shark populations in no-take and fished sites, across a 3 to 80 m depth gradient. Overall, nine species of sharks from five families were recorded; Carcharhinus melanopterus was the most abundant species. PERMANOVA analysis showed no statistical difference between areas nor zoning status; likely due to the relatively recent designation of the Marine Protected Areas (MPAs) and inconsistent management/enforcement. -

American Samoa Sharks and Rays
American Samoa Sharks and Rays Climate Change Vulnerability Assessment Summary An Important Note About this Document: This document represents an initial evaluation of vulnerability for sharks and rays based on workshop input and existing information. The aim of this document is to expand understanding of species vulnerability to changing climate conditions, and to provide a foundation for developing appropriate adaptation responses. Species Description Common species of sharks in American Samoa include the blacktip reef shark (Carcharhinus melanopterus) and the whitetip reef shark (Triaenodon obesus). Other rarer species include hammerhead sharks, tiger sharks (Galeocerdo cuvier), and whale sharks (Rhincodon typus).1 The most common species of rays in American Photo: NOAA Photo Library Samoa are Eagle rays (Myliobatidae sp.). Sharks and rays are generally rare across Indo-Pacific coral reef habitats, are not targeted by fishermen, and have been protected from catch and possession since 2012.1,2 Blacktip and whitetip reef sharks are commonly found in nearshore waters and sighted while diving, snorkeling, and swimming. Hammerhead sharks are known to give birth in Pago Pago Harbor, while few tiger sharks have been caught around Tutuila.1 Increased sea surface temperatures may affect shark and ray ranges, prey availability, and embryonic development.3 Extreme precipitation events can also diminish their ability to effectively use their sense of smell and electroreception to locate prey.3 Species Vulnerability Low-Moderate Vulnerability Low High Mod Confidence Sharks and rays were evaluated have low to moderate vulnerability by workshop participants due to climate and non-climate stressors such as ocean acidification, sea surface temperature, and changes in currents and wind. -

And the Whitetip Reef Shark Triaenodon Obesus in Tropical Waters
This file is part of the following reference: Robbins, William D. (2006) Abundance, demography and population structure of the grey reef shark (Carcharhinus amblyrhynchos) and the white tip reef shark (Triaenodon obesus) (Fam. Charcharhinidae). PhD thesis, James Cook University. Access to this file is available from: http://eprints.jcu.edu.au/2096 ABUNDANCE, DEMOGRAPHY AND POPULATION STRUCTURE OF THE GREY REEF SHARK (CARCHARHINUS AMBLYRHYNCHOS) AND THE WHITETIP REEF SHARK (TRIAENODON OBESUS) (FAM. CARCHARHINIDAE) Thesis submitted by William David ROBBINS B.Sc (Hons) April 2006 For the degree of Doctor of Philosophy in Marine Biology School of Marine Biology and Aquaculture James Cook University STATEMENT OF ACCESS I, the undersigned author of this work, understand that James Cook University will make this thesis available within the University Library, and via the Australian Digital Theses network, for use elsewhere. I declare that the electronic copy of this thesis provided to the James Cook University library is an accurate copy of the print thesis submitted, within the limits of the technology available. I understand that, as an unpublished work, a thesis has significant protection under the Copyright Act and; All users consulting this thesis must agree not to copy or closely paraphrase it in whole or in part without the written consent of the author; and to make proper public written acknowledgement for any assistance which they obtain from it. They must also agree to obtain prior written consent from the author before use or distribution of all or part of this thesis within 12 months of its award by James Cook University. -

And Their Functional, Ecological, and Evolutionary Implications
DePaul University Via Sapientiae College of Science and Health Theses and Dissertations College of Science and Health Spring 6-14-2019 Body Forms in Sharks (Chondrichthyes: Elasmobranchii), and Their Functional, Ecological, and Evolutionary Implications Phillip C. Sternes DePaul University, [email protected] Follow this and additional works at: https://via.library.depaul.edu/csh_etd Part of the Biology Commons Recommended Citation Sternes, Phillip C., "Body Forms in Sharks (Chondrichthyes: Elasmobranchii), and Their Functional, Ecological, and Evolutionary Implications" (2019). College of Science and Health Theses and Dissertations. 327. https://via.library.depaul.edu/csh_etd/327 This Thesis is brought to you for free and open access by the College of Science and Health at Via Sapientiae. It has been accepted for inclusion in College of Science and Health Theses and Dissertations by an authorized administrator of Via Sapientiae. For more information, please contact [email protected]. Body Forms in Sharks (Chondrichthyes: Elasmobranchii), and Their Functional, Ecological, and Evolutionary Implications A Thesis Presented in Partial Fulfilment of the Requirements for the Degree of Master of Science June 2019 By Phillip C. Sternes Department of Biological Sciences College of Science and Health DePaul University Chicago, Illinois Table of Contents Table of Contents.............................................................................................................................ii List of Tables..................................................................................................................................iv -

Species Composition of Elasmobranchs in the Surface and Subsurface Gillnet Operation in the Northern Arabian Sea
__________________________________________________IOTC-2019-WPEB15-13 Species composition of elasmobranchs in the surface and subsurface gillnet operation in the Northern Arabian Sea Muhammad Moazzam WWF-Pakistan, Karachi, Pakistan ([email protected]) Abstract Sharks form important part of bycatch of the tuna gillnet operations in Pakistan. WWF- Pakistan introduced subsurface gillnetting in 2014 in which gillnet are placed 1.4 to 2 m below the sea surface. Fishing fleet engaged in tuna gillnetting adopted subsurface gillnetting and by January 2016 entire tuna fleet was converted in subsurface gillnetting. Catch of endangered, threatened and protected (ETP) species such as dolphins and sea turtles were observed to be much lower in subsurface gillnet as compared to surface operations. Sharks are among the other ETP species whose catches were dropped in subsurface gillnet as compared to surface operations. It was observed that overall shark catches were 15.06 % lower in the subsurface gillnet operation as compared to surface placement of gillnets. A marked seasonality was observed in case of dominating species including mako and silky shark. Catches of mako sharks was observed to be about 8.65 % higher in subsurface gillnets as compared to surface gillnets. Introduction Sharks are considered as an important bycatch group of tuna gillnet fishing in Pakistan and other part of the Arabian Sea (Koya, 2018; Shahifar, 2018, Khan, 2013; Moazzam, 2013; Shahid et al., 2015, 2016). In Pakistan, gillnets consisting of monofilament and multifilament are used for catching tuna and tuna like species. Monofilament net is mainly used for catching neritic tuna in coastal waters whereas multifilament nylon nets are used for catching longtail tuna (Thunnus tonggol), yellowfin tuna (Thunnus albacares) and skipjack tuna (Katsuwonus pelamis) in the offshore waters. -

17 Reef Sharks (Carcharhinidae)
#17 Reef sharks (Carcharhinidae) claspers (in males) Sicklefi n lemon shark Blacktip shark (Negaprion acutidens) (Carcharhinus limbatus) Whitetip reef shark (Triaenodon obesus) Grey reef shark Blacktip reef shark (Carcharhinus amblyrhynchos) (Carcharhinus melanopterus) Species & Distribution Habitats & Feeding In the Pacific, inshore species caught by coastal communities Generally, small reef sharks prefer shallow, inshore areas for food include several species of small requiem sharks including reef flats and coral reefs. Young sharks may live (family Carcharhinidae). in inshore areas that offer plentiful food. Most species remain in particular areas whereas blacktip sharks move Common species include the blacktip reef shark, Carcharhinus over considerable distances. melanopterus, the blacktip shark, Carcharhinus limbatus, the grey reef shark, Carcharhinus amblyrhynchos, the sicklefin Most reef sharks hunt alone but can form feeding frenzies lemon shark, Negaprion acutidens, and the whitetip reef when people spearfish or gut fish in the water. They eat fish shark, Triaenodon obesus. such as sardines, mullets, mackerels, trevallies and emperors as well as octopuses and shrimps. Whitetip reef sharks often These smaller species have a wide distribution in the rest under coral during the day and hunt at night. IndoPacific and, other than the lemon shark that grows to 3 metres, most reach a size of about 2 metres. Several other Small reef sharks are eaten by larger fish including sharks larger and more dangerous species, including tiger sharks and groupers. and bull sharks, are caught, mainly by commercial fishers. Most of these sharks swim continually to obtain oxygen from water flowing over their gills; the whitetip reef shark, however, can pump water over its gills and lie motionless on the sea floor. -
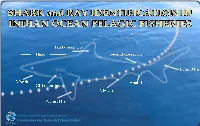
Sharks and Rays That Interact with Tuna Fisheries in the Indian Ocean
Indian Ocean Tuna Commission Commission des Thons de l’Océan Indien These identification cards are produced by the Indian Ocean Tuna Commission (IOTC), in collaboration with the Secretariat of the Pacific Community (SPC), to help improve catch data and statistics on sharks and rays that interact with tuna fisheries in the Indian Ocean. With a better understanding of shark stocks and with better statistics, regional fisheries managers can ensure that sharks and rays are fished in a sustainable manner in the Indian Ocean. The most likely users of the cards are fisheries observers, samplers, fishing masters and crew on board fishing vessels targeting tuna, tuna-like species and sharks in the Indian Ocean. Fisheries training institutions and fishing communities are other potential users. This publication was made possible through financial support provided by the <partner>. For further information contact: Indian Ocean Tuna Commission Le Chantier Mall PO Box 1011, Victoria, Seychelles Phone: +248 422 54 94 Fax: +248 422 43 64 Email: [email protected] Website: http://www.iotc.org This publication is based on that developed by the Secretariat of the Pacific Community entitled «Shark identication in Pacific tropical offshore fisheries» (conception: M. Blanc / design: A.Desurmont and T. Luciani) Layout: Julien Million and Jipé Le-Bars. Scientific advice: Dr. Evgeny Romanov and Dr. Charles Anderson. Acknowledgements to Dr. Rui Coelho, Dr. Bernard Seret and Dr. David Wilson for their valuable inputs. Illustrations © R.Swainston/anima.net.au. One shark illustration - Odontaspis noronhai - by L. Hata reproduced courtesy of SPC. Four ray illustrations – Manta alfredi, Manta birostris, Mobula mobular, Mobula tarapacana – by J.F. -

Complete List of Exempt Ray and Shark Species
OREGON DEPARTMENT OF FISH AND WILDLFE Shark and ray species exempt from wildlife trafficking prohibitions because they are subject to federal management plan The Oregon Department of Fish and Wildlife (ODFW) has developed a list of shark and ray species that are subject to a federal management plan and therefore exempt from the prohibitions of ORS 498.022 (Table 1). The North Pacific Fishery Management Council’s (NPFMC) Gulf of Alaska Groundfish (GOAGF) Fishery Management Plan (FMP) includes all shark species found in the management area. For this FMP, ODFW included those species of shark with reported distributions that overlap the GOA-GF management in Table 1. Several Western Pacific Fishery Management Council (WPFMC) Fishery Ecosystem Plans (FEPs) include entire taxonomic families, but individual species within those families may not occur in the fishery management area. For these family level listings, ODFW examined the distribution for each species within each family and included those that have reported distributions overlapping one or more WPFMC management areas in Table 1. Acronyms ASA – America Samoa Archipelago CAHMS – Consolidated Atlantic Highly Migratory Species GF – Groundfish GOAGF – Gulf of Alaska Groundfish HA – Hawaii Archipelago HMS – Highly Migratory Species MA – Marina Archipelago MAFMC – Mid-Atlantic Fishery Management Council MSA – Magnuson-Stevens Fishery Conservation and Management Act NPFMC – North Pacific Fishery Management Council ODFW – Oregon Department of Fish and Wildlife ORS – Oregon Revised Statutes PFMC – Pacific Fishery Management Council PP – Pacific Pelagics PRIA – Pacific Remote Islands Area SD – Spiny Dogfish SoC – Secretary of State WPFMC – Western Pacific Fishery Management Council August 16, 2017 OREGON DEPARTMENT OF FISH AND WILDLFE Table 1. -

Whitetip Reef Shark Facts
Please Visit Our Website At: http://www.SharkSider.com The Top 50 COOLEST Sharks in the World! Written By: Lisa Evans http://www.SharkSider.com Please Visit Our Website At: http://www.SharkSider.com Copyright © 2014 by Rogers Concepts, LLC. www.SharkSider.com This eBook was created by SharkSider.com. Please visit our website to learn even more about sharks including additional images, videos, and facts. Image Credits: All images used in this eBook were taken from various “free use” sources from the public domain. Images are Copyright free and may be altered or used for any purpose, even commercially. Permission is NOT required to use images contained in this eBook. U.S. Copyright protects all written content in this eBook. No written material contained in this eBook may be used without the written consent of Rogers Concepts, LLC. For information about using written content contained within this eBook or for media inquiries, please contact Mike Rogers at: [email protected] Please Save Our Sharks – Boycott Shark Fin Soup! Shark Fin Soup is destroying the shark population. Did you know up to 100-MILLION sharks are illegally removed from the Ocean’s every year, just so their fins can be used for soup? Making a difference is easy. If you are at a restaurant and see Shark Fin Soup on the menu, voice your concern and do not give them any further business. As consumers, we can make a difference. Please visit www.SharkSider.com/shark-fin-soup.html to learn more and to help stop this needless practice. Please Visit Our Website At: http://www.SharkSider.com Dedicated to Young and Old Shark Fans Everywhere Thank you so much for purchasing The Top 50 COOLEST Sharks in the World! It really means a lot to us and we just know you’re going to love it. -

AN OVERVIEW of SHARK UTILISATION in the CORAL TRIANGLE REGION Written By
WORKING TOGETHER FOR SUSTAINABLE SHARK FISHERIES AN OVERVIEW OF SHARK UTILISATION IN THE CORAL TRIANGLE REGION Written by Mary Lack Director, Shellack Pty Ltd Glenn Sant Fisheries Programme Leader, TRAFFIC & Senior Fellow, ANCORS Published in September 2012 This report can be downloaded from wwf.panda.org/coraltriangle Citation Lack M. and Sant G. (2012). An overview of shark utilisation in the Coral Triangle region. TRAFFIC &WWF. Photo cover © naturepl.com / Jeff Rotman / WWF-Canon Thanks to the Rufford Lang Foundation for supporting the development of this publication 2 An Overview Of Shark Utilisation In The Coral Triangle Region ACRONYMS ASEAN Association of Southeast Asian Nations BFAR Bureau of Fisheries and Aquatic Resources (the Philippines) CCSBT Commission for the Conservation of Southern Bluefin Tuna CITES Convention on International Trade in Endangered Species of Wild Fauna and Flora CMM Conservation and Management Measure CMS Convention on Migratory Species of Wild Animals CNP Co-operating Non-Contracting party COFI Committee on Fisheries (of FAO) CoP Conference of the Parties (to CITES) EEZ Exclusive Economic Zone EU European Union FAO Food and Agriculture Organization of the United Nations IOTC Indian Ocean Tuna Commission IPOA-Sharks International Plan of Action for the Conservation and Management of Sharks IUU Illegal, Unreported and Unregulated (fishing) MoU Memorandum of Understanding on the Conservation of Migratory Sharks (CMS) nei Not elsewhere included NPOA-Sharks National Plan of Action for the Conservation and -
Shark and Ray By-Catch
ASSESSING SHARK AND RAY BYCATCH IN INDONESIAN DEEPWATER SNAPPER- GROUPER FISHERIES Report on data analysis January 2017 - December 2018 Project ID: P104884 Prepared for The Nature Conservancy Indonesia by Steven Lindfield and Vanessa Jaiteh Coral Reef Research Foundation October, 2019 ASSESSING SHARK AND RAY BYCATCH IN INDONESIAN SNAPPER-GROUPER FISHERIES 1. BACKGROUND AND OBJECTIVES The purpose of this work is to assess shark and ray bycatch in Indonesian deep-water snapper and grouper fisheries. Since 2014, The Nature Conservancy (TNC) has been working with a number of Indonesian deep-slope (50-500 m) dropline and demersal longline fisheries that target various snapper, grouper and emperorfish species. These fisheries are currently being monitored using a Crew-Operated Data Recording System (CODRS) which provides photographs of all target and non-target catch. This monitoring program is organized through TNC’s Indonesia field office to support the development and adoption of data-poor stock assessment approaches with involvement of the private sector in data collection. Following the application of CODRS, TNC has gained information not only on the snapper, grouper, and emperorfish fishery but also its shark and ray bycatch. Globally, one quarter of all shark and ray species are currently threatened with extinction mainly due to their capture in target fisheries and as bycatch in other fisheries. In the last decade, government and non-government organizations have widely adopted conservation efforts to restore threatened shark populations, but these efforts are often hindered by a lack of comprehensive and accurate data. This report assesses shark and ray bycatch through the development of a species list (according to species-level identification of the catch based on photographs that the crew take aboard fishing vessels).