SHANK Proteins: Roles at the Synapse and in Autism Spectrum Disorder
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Exploring the Mechanisms Underlying Excitation/Inhibition Imbalance in Human Ipsc-Derived Models of ASD Lorenza Culotta1,3 and Peter Penzes1,2,3*
Culotta and Penzes Molecular Autism (2020) 11:32 https://doi.org/10.1186/s13229-020-00339-0 REVIEW Open Access Exploring the mechanisms underlying excitation/inhibition imbalance in human iPSC-derived models of ASD Lorenza Culotta1,3 and Peter Penzes1,2,3* Abstract Autism spectrum disorder (ASD) is a range of neurodevelopmental disorders characterized by impaired social interaction and communication, and repetitive or restricted behaviors. ASD subjects exhibit complex genetic and clinical heterogeneity, thus hindering the discovery of pathophysiological mechanisms. Considering that several ASD-risk genes encode proteins involved in the regulation of synaptic plasticity, neuronal excitability, and neuronal connectivity, one hypothesis that has emerged is that ASD arises from a disruption of the neuronal network activity due to perturbation of the synaptic excitation and inhibition (E/I) balance. The development of induced pluripotent stem cell (iPSC) technology and recent advances in neuronal differentiation techniques provide a unique opportunity to model complex neuronal connectivity and to test the E/I hypothesis of ASD in human-based models. Here, we aim to review the latest advances in studying the different cellular and molecular mechanisms contributing to E/I balance using iPSC-based in vitro models of ASD. Keywords: Autism spectrum disorder, Induced pluripotent stem cell, Excitation/inhibition balance Background ASD can be classified into syndromic and non- Autism spectrum disorder (ASD) represents a spectrum of syndromic forms [4–7]. Syndromic ASD accounts for a early-onset neurodevelopmental disorders characterized by small percentage of total ASD cases; it typically occurs persistent deficits in social interaction and communication, with a clinical presentation in association with secondary as well as repetitive patterns of behavior and restricted inter- phenotypes and/or dysmorphic features [6]. -

Identification and Functional Characterization of Rare SHANK2
OPEN Molecular Psychiatry (2015) 20, 1489–1498 © 2015 Macmillan Publishers Limited All rights reserved 1359-4184/15 www.nature.com/mp ORIGINAL ARTICLE Identification and functional characterization of rare SHANK2 variants in schizophrenia S Peykov1, S Berkel1, M Schoen2, K Weiss3, F Degenhardt4, J Strohmaier5, B Weiss1, C Proepper2, G Schratt3, MM Nöthen4,5, TM Boeckers2, M Rietschel6 and GA Rappold1,7 Recent genetic data on schizophrenia (SCZ) have suggested that proteins of the postsynaptic density of excitatory synapses have a role in its etiology. Mutations in the three SHANK genes encoding for postsynaptic scaffolding proteins have been shown to represent risk factors for autism spectrum disorders and other neurodevelopmental disorders. To address if SHANK2 variants are associated with SCZ, we sequenced SHANK2 in 481 patients and 659 unaffected individuals. We identified a significant increase in the number of rare (minor allele frequencyo1%) SHANK2 missense variants in SCZ individuals (6.9%) compared with controls (3.9%, P = 0.039). Four out of fifteen non-synonymous variants identified in the SCZ cohort (S610Y, R958S, P1119T and A1731S) were selected for functional analysis. Overexpression and knockdown-rescue experiments were carried out in cultured primary hippocampal neurons with a major focus on the analysis of morphological changes. Furthermore, the effect on actin polymerization in fibroblast cell lines was investigated. All four variants revealed functional impairment to various degrees, as a consequence of alterations in spine volume and clustering at synapses and an overall loss of presynaptic contacts. The A1731S variant was identified in four unrelated SCZ patients (0.83%) but not in any of the sequenced controls and public databases (P = 4.6 × 10 − 5). -

Lysine-Specific Demethylase 1 Is Strongly Expressed in Poorly Differentiated Neuroblastoma: Implications for Therapy
Published OnlineFirst February 17, 2009; DOI: 10.1158/0008-5472.CAN-08-1735 Research Article Lysine-Specific Demethylase 1 Is Strongly Expressed in Poorly Differentiated Neuroblastoma: Implications for Therapy Johannes H. Schulte,1 Soyoung Lim,3 Alexander Schramm,1 Nicolaus Friedrichs,3 Jan Koster,4 Rogier Versteeg,4 Ingrid Ora,4,5 Kristian Pajtler,1 Ludger Klein-Hitpass,2 Steffi Kuhfittig-Kulle,1 Eric Metzger,6 Roland Schu¨le,6 Angelika Eggert,1 Reinhard Buettner,3 and Jutta Kirfel3 1Department of Paediatric Oncology and Hematology, University Children’s Hospital Essen; 2Institute of Cell Biology, University Hospital of Essen, Essen, Germany; 3Institute of Pathology, University of Bonn, Bonn, Germany; 4Department of Human Genetics, Academic Medical Center, University of Amsterdam, Amsterdam, the Netherlands; 5Department of Pediatric Oncology and Hematology, Lund University Hospital, Lund, Sweden; 6Center for Clinical Research, Freiburg University Medical Center, Freiburg, Germany Abstract often fatally progresses regardless of multimodal therapy (1, 2). Aberrant epigenetic changes in DNA methylation and histone Therefore, the identification of novel drug targets and development acetylation are hallmarks of most cancers, whereas histone of new therapeutic options are urgently needed. Patterns methylation was previously considered to be irreversible and characteristic of aggressive neuroblastoma have been identified less versatile. Recently, several histone demethylases were via high-throughput analysis, including expression profiling (3, 4) identified catalyzing the removal of methyl groups from histone and array CGH (5–7), with NMYC amplification, 1p36 and 11q H3 lysine residues and thereby influencing gene expression. deletion (8), and 17q gain being the most prominent chromosomal Neuroblastomas continue to remain a clinical challenge alterations. -

Structural Variation Targets Neurodevelopmental Genes and Identifies SHANK2 As a Tumor
bioRxiv preprint doi: https://doi.org/10.1101/572248; this version posted March 13, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Structural Variation in Neuroblastoma Lopez, Conkrite, et al. Structural variation targets neurodevelopmental genes and identifies SHANK2 as a tumor suppressor in neuroblastoma Gonzalo Lopez1,2+, Karina L. Conkrite1,2+, Miriam Doepner1,2, Komal S. Rathi3, Apexa Modi1,2,10, Zalman Vaksman1-3, Lance M. Farra1,2, Eric Hyson1,2, Moataz Noureddine1,2, Jun S. Wei4, Malcolm A. Smith9, Shahab Asgharzadeh5,6, Robert C. Seeger5,6, Javed Khan4, Jaime Guidry Auvil8, Daniela S. Gerhard8, John M. Maris1-2,10,11, Sharon J. Diskin1-3,10,11* 1Division of Oncology, Children’s Hospital of Philadelphia, Philadelphia, PA, USA. 2Center for Childhood Cancer Research, Children’s Hospital of Philadelphia, Philadelphia, PA, USA. 3Department of Biomedical and Health Informatics, Children’s Hospital of Philadelphia, Philadelphia, PA, USA. 4Oncogenomics Section, Genetics Branch, Center for Cancer Research, National Cancer Institute, Bethesda, MD, USA 5Division of Hematology, Oncology and Blood and Marrow Transplantation, Keck School of Medicine of the University of Southern California, Los Angeles, CA, USA. 6The Saban Research Institute, Children’s Hospital of Los Angeles, Los Angeles, CA, USA. 7Department of Pediatrics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA. 8Office of Cancer Genomics, National Cancer Institute, Bethesda, MD, USA. 9Cancer Therapy Evaluation Program, National Cancer Institute, Bethesda, MD, USA. 10Genomics and Computational Biology, Biomedical Graduate Studies, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA. -

Homer1 Promotes Dendritic Spine Growth Through Ankyrin-G and Its Loss Reshapes the Synaptic Proteome
Molecular Psychiatry (2021) 26:1775–1789 https://doi.org/10.1038/s41380-020-00991-1 ARTICLE Homer1 promotes dendritic spine growth through ankyrin-G and its loss reshapes the synaptic proteome 1 1 2 1,5 2 Sehyoun Yoon ● Nicolas H. Piguel ● Natalia Khalatyan ● Leonardo E. Dionisio ● Jeffrey N. Savas ● Peter Penzes 1,3,4 Received: 22 February 2020 / Revised: 24 November 2020 / Accepted: 7 December 2020 / Published online: 4 January 2021 © The Author(s), under exclusive licence to Springer Nature Limited part of Springer Nature 2021. This article is published with open access Abstract Homer1 is a synaptic scaffold protein that regulates glutamatergic synapses and spine morphogenesis. HOMER1 knockout (KO) mice show behavioral abnormalities related to psychiatric disorders, and HOMER1 has been associated with psychiatric disorders such as addiction, autism disorder (ASD), schizophrenia (SZ), and depression. However, the mechanisms by which it promotes spine stability and its global function in maintaining the synaptic proteome has not yet been fully investigated. Here, we used computational approaches to identify global functions for proteins containing the Homer1-interacting PPXXF motif within the postsynaptic compartment. Ankyrin-G was one of the most topologically important nodes in the postsynaptic peripheral 1234567890();,: 1234567890();,: membrane subnetwork, and we show that one of the PPXXF motifs, present in the postsynaptically-enriched 190 kDa isoform of ankyrin-G (ankyrin-G 190), is recognized by the EVH1 domain of Homer1. We use proximity ligation combined with super- resolution microscopy to map the interaction of ankyrin-G and Homer1 to distinct nanodomains within the spine head and correlate them with spine head size. -

8P23.2-Pter Microdeletions: Seven New Cases Narrowing the Candidate Region and Review of the Literature
G C A T T A C G G C A T genes Article 8p23.2-pter Microdeletions: Seven New Cases Narrowing the Candidate Region and Review of the Literature Ilaria Catusi 1,* , Maria Garzo 1 , Anna Paola Capra 2 , Silvana Briuglia 2 , Chiara Baldo 3 , Maria Paola Canevini 4 , Rachele Cantone 5, Flaviana Elia 6, Francesca Forzano 7, Ornella Galesi 8, Enrico Grosso 5, Michela Malacarne 3, Angela Peron 4,9,10 , Corrado Romano 11 , Monica Saccani 4 , Lidia Larizza 1 and Maria Paola Recalcati 1 1 Istituto Auxologico Italiano, IRCCS, Laboratory of Medical Cytogenetics and Molecular Genetics, 20145 Milan, Italy; [email protected] (M.G.); [email protected] (L.L.); [email protected] (M.P.R.) 2 Department of Biomedical, Dental, Morphological and Functional Imaging Sciences, University of Messina, 98100 Messina, Italy; [email protected] (A.P.C.); [email protected] (S.B.) 3 UOC Laboratorio di Genetica Umana, IRCCS Istituto Giannina Gaslini, 16147 Genova, Italy; [email protected] (C.B.); [email protected] (M.M.) 4 Child Neuropsychiatry Unit—Epilepsy Center, Department of Health Sciences, ASST Santi Paolo e Carlo, San Paolo Hospital, Università Degli Studi di Milano, 20142 Milan, Italy; [email protected] (M.P.C.); [email protected] (A.P.); [email protected] (M.S.) 5 Medical Genetics Unit, Città della Salute e della Scienza University Hospital, 10126 Turin, Italy; [email protected] (R.C.); [email protected] (E.G.) 6 Unit of Psychology, Oasi Research Institute-IRCCS, -

Deciphering the Molecular Profile of Plaques, Memory Decline And
ORIGINAL RESEARCH ARTICLE published: 16 April 2014 AGING NEUROSCIENCE doi: 10.3389/fnagi.2014.00075 Deciphering the molecular profile of plaques, memory decline and neuron loss in two mouse models for Alzheimer’s disease by deep sequencing Yvonne Bouter 1†,Tim Kacprowski 2,3†, Robert Weissmann4, Katharina Dietrich1, Henning Borgers 1, Andreas Brauß1, Christian Sperling 4, Oliver Wirths 1, Mario Albrecht 2,5, Lars R. Jensen4, Andreas W. Kuss 4* andThomas A. Bayer 1* 1 Division of Molecular Psychiatry, Georg-August-University Goettingen, University Medicine Goettingen, Goettingen, Germany 2 Department of Bioinformatics, Institute of Biometrics and Medical Informatics, University Medicine Greifswald, Greifswald, Germany 3 Department of Functional Genomics, Interfaculty Institute for Genetics and Functional Genomics, University Medicine Greifswald, Greifswald, Germany 4 Human Molecular Genetics, Department for Human Genetics of the Institute for Genetics and Functional Genomics, Institute for Human Genetics, University Medicine Greifswald, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany 5 Institute for Knowledge Discovery, Graz University of Technology, Graz, Austria Edited by: One of the central research questions on the etiology of Alzheimer’s disease (AD) is the Isidro Ferrer, University of Barcelona, elucidation of the molecular signatures triggered by the amyloid cascade of pathological Spain events. Next-generation sequencing allows the identification of genes involved in disease Reviewed by: Isidro Ferrer, University of Barcelona, processes in an unbiased manner. We have combined this technique with the analysis of Spain two AD mouse models: (1) The 5XFAD model develops early plaque formation, intraneu- Dietmar R. Thal, University of Ulm, ronal Ab aggregation, neuron loss, and behavioral deficits. (2)TheTg4–42 model expresses Germany N-truncated Ab4–42 and develops neuron loss and behavioral deficits albeit without plaque *Correspondence: formation. -

LGI1–ADAM22–MAGUK Configures Transsynaptic Nanoalignment for Synaptic Transmission and Epilepsy Prevention
LGI1–ADAM22–MAGUK configures transsynaptic nanoalignment for synaptic transmission and epilepsy prevention Yuko Fukataa,b,1, Xiumin Chenc,d,1, Satomi Chikenb,e, Yoko Hiranoa,f, Atsushi Yamagatag, Hiroki Inahashia, Makoto Sanboh, Hiromi Sanob,e, Teppei Gotoh, Masumi Hirabayashib,h, Hans-Christian Kornaui,j, Harald Prüssi,k, Atsushi Nambub,e, Shuya Fukail, Roger A. Nicollc,d,2, and Masaki Fukataa,b,2 aDivision of Membrane Physiology, Department of Molecular and Cellular Physiology, National Institute for Physiological Sciences, National Institutes of Natural Sciences, Aichi 444-8787, Japan; bDepartment of Physiological Sciences, School of Life Science, SOKENDAI (The Graduate University for Advanced Studies), Aichi 444-8585, Japan; cDepartment of Cellular and Molecular Pharmacology, University of California, San Francisco, CA 94158; dDepartment of Physiology, University of California, San Francisco, CA 94158; eDivision of System Neurophysiology, Department of System Neuroscience, National Institute for Physiological Sciences, National Institutes of Natural Sciences, Aichi 444-8585, Japan; fDepartment of Pediatrics, Graduate School of Medicine, The University of Tokyo, Tokyo 113-8655, Japan; gLaboratory for Protein Functional and Structural Biology, RIKEN Center for Biosystems Dynamics Research, Kanagawa 230-0045, Japan; hCenter for Genetic Analysis of Behavior, National Institute for Physiological Sciences, National Institutes of Natural Sciences, Okazaki 444-8787, Japan; iGerman Center for Neurodegenerative Diseases (DZNE), 10117 Berlin, Germany; jNeuroscience Research Center, Cluster NeuroCure, Charité-Universitätsmedizin Berlin, 10117 Berlin, Germany; kDepartment of Neurology and Experimental Neurology, Charité-Universitätsmedizin Berlin, 10117 Berlin, Germany; and lDepartment of Chemistry, Graduate School of Science, Kyoto University, 606-8502 Kyoto, Japan Contributed by Roger A. Nicoll, December 1, 2020 (sent for review October 29, 2020; reviewed by David S. -
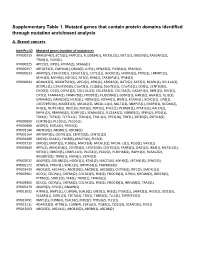
Supplementary Table 1. Mutated Genes That Contain Protein Domains Identified Through Mutation Enrichment Analysis
Supplementary Table 1. Mutated genes that contain protein domains identified through mutation enrichment analysis A. Breast cancers InterPro ID Mutated genes (number of mutations) IPR000219 ARHGEF4(2), ECT2(1), FARP1(1), FLJ20184(1), MCF2L2(1), NET1(1), OBSCN(5), RASGRF2(2), TRAD(1), VAV3(1) IPR000225 APC2(2), JUP(1), KPNA5(2), SPAG6(1) IPR000357 ARFGEF2(2), CMYA4(1), DRIM(2), JUP(1), KPNA5(2), PIK3R4(1), SPAG6(1) IPR000533 AKAP9(2), C10orf39(1), C20orf23(1), CUTL1(1), HOOK1(1), HOOK3(1), KTN1(2), LRRFIP1(3), MYH1(3), MYH9(2), NEF3(1), NF2(1), RSN(1), TAX1BP1(1), TPM4(1) IPR000694 ADAM12(3), ADAMTS19(1), APC2(2), APXL(1), ARID1B(1), BAT2(2), BAT3(1), BCAR1(1), BCL11A(2), BCORL1(1), C14orf155(3), C1orf2(1), C1QB(1), C6orf31(1), C7orf11(1), CD2(1), CENTD3(3), CHD5(3), CIC(3), CMYA1(2), COL11A1(3), COL19A1(2), COL7A1(3), DAZAP1(1), DBN1(3), DVL3(1), EIF5(1), FAM44A(1), FAM47B(1), FHOD1(1), FLJ20584(1), G3BP2(2), GAB1(2), GGA3(1), GLI1(3), GPNMB(2), GRIN2D(3), HCN3(1), HOXA3(2), HOXA4(1), IRS4(1), KCNA5(1), KCNC2(1), LIP8(1), LOC374955(1), MAGEE1(2), MICAL1(2), MICAL‐L1(1), MLLT2(1), MMP15(1), N4BP2(1), NCOA6(2), NHS(1), NUP214(3), ODZ1(3), PER1(2), PER2(1), PHC1(1), PLXNB1(1), PPM1E(2), RAI17(2), RAPH1(2), RBAF600(2), SCARF2(1), SEMA4G(1), SLC16A2(1), SORBS1(1), SPEN(2), SPG4(1), TBX1(1), TCF1(2), TCF7L1(1), TESK1(1), THG‐1(1), TP53(18), TRIF(1), ZBTB3(2), ZNF318(2) IPR000909 CENTB1(2), PLCB1(1), PLCG1(1) IPR000998 AEGP(3), EGFL6(2), PRSS7(1) IPR001140 ABCB10(2), ABCB6(1), ABCB8(2) IPR001164 ARFGAP3(1), CENTB1(2), CENTD3(3), CENTG1(2) IPR001589 -

The Human Gene Connectome As a Map of Short Cuts for Morbid Allele Discovery
The human gene connectome as a map of short cuts for morbid allele discovery Yuval Itana,1, Shen-Ying Zhanga,b, Guillaume Vogta,b, Avinash Abhyankara, Melina Hermana, Patrick Nitschkec, Dror Friedd, Lluis Quintana-Murcie, Laurent Abela,b, and Jean-Laurent Casanovaa,b,f aSt. Giles Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, NY 10065; bLaboratory of Human Genetics of Infectious Diseases, Necker Branch, Paris Descartes University, Institut National de la Santé et de la Recherche Médicale U980, Necker Medical School, 75015 Paris, France; cPlateforme Bioinformatique, Université Paris Descartes, 75116 Paris, France; dDepartment of Computer Science, Ben-Gurion University of the Negev, Beer-Sheva 84105, Israel; eUnit of Human Evolutionary Genetics, Centre National de la Recherche Scientifique, Unité de Recherche Associée 3012, Institut Pasteur, F-75015 Paris, France; and fPediatric Immunology-Hematology Unit, Necker Hospital for Sick Children, 75015 Paris, France Edited* by Bruce Beutler, University of Texas Southwestern Medical Center, Dallas, TX, and approved February 15, 2013 (received for review October 19, 2012) High-throughput genomic data reveal thousands of gene variants to detect a single mutated gene, with the other polymorphic genes per patient, and it is often difficult to determine which of these being of less interest. This goes some way to explaining why, variants underlies disease in a given individual. However, at the despite the abundance of NGS data, the discovery of disease- population level, there may be some degree of phenotypic homo- causing alleles from such data remains somewhat limited. geneity, with alterations of specific physiological pathways under- We developed the human gene connectome (HGC) to over- come this problem. -

Rare Gene Deletions in Genetic Generalized and Rolandic Epilepsies
RESEARCH ARTICLE Rare gene deletions in genetic generalized and Rolandic epilepsies Kamel Jabbari1,2☯, Dheeraj R. Bobbili3☯, Dennis Lal1,4,5,6, Eva M. Reinthaler7, Julian Schubert8, Stefan Wolking8, Vishal Sinha9, Susanne Motameny1, Holger Thiele1, Amit Kawalia1, Janine AltmuÈ ller1,10, Mohammad Reza Toliat1, Robert Kraaij11, Jeroen van Rooij11, Andre G. Uitterlinden11, M. Arfan Ikram12, EuroEPINOMICS CoGIE Consortium¶, Federico Zara13, Anna-Elina Lehesjoki14,15, Roland Krause3, Fritz Zimprich7, Thomas Sander1, Bernd A. Neubauer16, Patrick May3, Holger Lerche8, Peter NuÈrnberg1,17,18* a1111111111 1 Cologne Center for Genomics, University of Cologne, Cologne, Germany, 2 Cologne Biocenter, Institute a1111111111 for Genetics, University of Cologne, Cologne, Germany, 3 Luxembourg Centre for Systems Biomedicine, a1111111111 University of Luxembourg, Esch-sur-Alzette, Luxembourg, 4 Psychiatric and Neurodevelopmental Genetics a1111111111 Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, United States of a1111111111 America, 5 Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, United States of America, 6 Stanley Center for Psychiatric Research, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, United States of America, 7 Department of Neurology, Medical University of Vienna, Vienna, Austria, 8 Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research, University of TuÈbingen, TuÈbingen, Germany, 9 Institute for Molecular Medicine FIMM, University of Helsinki, Helsinki, Finland, 10 Institute of Human Genetics, University of Cologne, OPEN ACCESS Cologne, Germany, 11 Department of Internal Medicine, Erasmus Medical Center, Rotterdam, the Netherlands, 12 Departments of Epidemiology, Neurology, and Radiology, Erasmus Medical Center, Citation: Jabbari K, Bobbili DR, Lal D, Reinthaler Rotterdam, The Netherlands, 13 Laboratory of Neurogenetics and Neuroscience, Institute G. -

A 29 Mainland Chinese Cohort of Patients with Phelan–Mcdermid
Xu et al. Orphanet J Rare Dis (2020) 15:335 https://doi.org/10.1186/s13023-020-01592-5 RESEARCH Open Access A 29 Mainland Chinese cohort of patients with Phelan–McDermid syndrome: genotype– phenotype correlations and the role of SHANK3 haploinsufciency in the important phenotypes Na Xu1†, Hui Lv2†, Tingting Yang1†, Xiujuan Du2, Yu Sun1, Bing Xiao1, Yanjie Fan1, Xiaomei Luo1, Yongkun Zhan1, Lili Wang1, Fei Li2* and Yongguo Yu1,3* Abstract Background: Phelan–McDermid syndrome (PMS) or 22q13 deletion syndrome is a rare developmental disorder characterized by hypotonia, developmental delay (DD), intellectual disability (ID), autism spectrum disorder (ASD) and dysmorphic features. Most cases are caused by 22q13 deletions encompassing many genes including SHANK3. Phenotype comparisons between patients with SHANK3 mutations (or deletions only disrupt SHANK3) and 22q13 deletions encompassing more than SHANK3 gene are lacking. Methods: A total of 29 Mainland China patients were clinically and genetically evaluated. Data were obtained from medical record review and a standardized medical history questionnaire, and dysmorphology evaluation was conducted via photographic evaluation. We analyzed 22q13 deletions and SHANK3 small mutations and performed genotype–phenotype analysis to determine whether neurological features and other important clinical features are responsible for haploinsufciency of SHANK3. Results: Nineteen patients with 22q13.3 deletions ranging in size from 34 kb to 8.7 Mb, one patient with terminal deletions and duplications, and nine patients with SHANK3 mutations were included. All mutations would cause loss-of function efect and six novel heterozygous variants, c.3838_3839insGG, c.3088delC, c.3526G > T, c.3372dupC, c.3120delC and c.3942delC, were frstly reported.