Deca-Durabolin 50 Mg/Ml Solution for Injection (Nandrolone Decanoate)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hormonal Treatment Strategies Tailored to Non-Binary Transgender Individuals
Journal of Clinical Medicine Review Hormonal Treatment Strategies Tailored to Non-Binary Transgender Individuals Carlotta Cocchetti 1, Jiska Ristori 1, Alessia Romani 1, Mario Maggi 2 and Alessandra Daphne Fisher 1,* 1 Andrology, Women’s Endocrinology and Gender Incongruence Unit, Florence University Hospital, 50139 Florence, Italy; [email protected] (C.C); jiska.ristori@unifi.it (J.R.); [email protected] (A.R.) 2 Department of Experimental, Clinical and Biomedical Sciences, Careggi University Hospital, 50139 Florence, Italy; [email protected]fi.it * Correspondence: fi[email protected] Received: 16 April 2020; Accepted: 18 May 2020; Published: 26 May 2020 Abstract: Introduction: To date no standardized hormonal treatment protocols for non-binary transgender individuals have been described in the literature and there is a lack of data regarding their efficacy and safety. Objectives: To suggest possible treatment strategies for non-binary transgender individuals with non-standardized requests and to emphasize the importance of a personalized clinical approach. Methods: A narrative review of pertinent literature on gender-affirming hormonal treatment in transgender persons was performed using PubMed. Results: New hormonal treatment regimens outside those reported in current guidelines should be considered for non-binary transgender individuals, in order to improve psychological well-being and quality of life. In the present review we suggested the use of hormonal and non-hormonal compounds, which—based on their mechanism of action—could be used in these cases depending on clients’ requests. Conclusion: Requests for an individualized hormonal treatment in non-binary transgender individuals represent a future challenge for professionals managing transgender health care. For each case, clinicians should balance the benefits and risks of a personalized non-standardized treatment, actively involving the person in decisions regarding hormonal treatment. -

(12) Patent Application Publication (10) Pub. No.: US 2014/0296.191 A1 PATEL Et Al
US 20140296.191A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0296.191 A1 PATEL et al. (43) Pub. Date: Oct. 2, 2014 (54) COMPOSITIONS OF PHARMACEUTICAL (52) U.S. Cl. ACTIVES CONTAINING DETHYLENE CPC ............... A61K 47/10 (2013.01); A61 K9/0019 GLYCOL MONOETHYLETHER OR OTHER (2013.01); A61 K9/0048 (2013.01); A61 K ALKYL DERVATIVES 45/06 (2013.01) USPC ........... 514/167: 514/177; 514/178: 514/450; (71) Applicant: THEMIS MEDICARE LIMITED, 514/334: 514/226.5: 514/449; 514/338; Mumbai (IN) 514/256; 514/570; 514/179; 514/174: 514/533; (72) Inventors: Dinesh Shantilal PATEL, Mumbai (IN); 514/629; 514/619 Sachin Dinesh PATEL, Mumbai (IN); Shashikant Prabhudas KURANI, Mumbai (IN); Madhavlal Govindlal (57) ABSTRACT PATEL, Mumbai (IN) (73) Assignee: THEMIS MEDICARE LIMITED, The present invention relates to pharmaceutical compositions Mumbai (IN) of various pharmaceutical actives, especially lyophilic and hydrophilic actives containing Diethylene glycol monoethyl (21) Appl. No.: 14/242,973 ether or other alkyl derivatives thereofas a primary vehicle and/or to pharmaceutical compositions utilizing Diethylene (22) Filed: Apr. 2, 2014 glycol monoethyl ether or other alkyl derivatives thereofas a primary vehicle or as a solvent system in preparation of Such (30) Foreign Application Priority Data pharmaceutical compositions. The pharmaceutical composi Apr. 2, 2013 (IN) ......................... 1287/MUMA2013 tions of the present invention are safe, non-toxic, exhibits enhanced physical stability compared to conventional formu Publication Classification lations containing such pharmaceutical actives and are Suit able for use as injectables for intravenous and intramuscular (51) Int. Cl. administration, as well as for use as a preformed solution/ A647/ (2006.01) liquid for filling in and preparation of capsules, tablets, nasal A6 IK 45/06 (2006.01) sprays, gargles, dermal applications, gels, topicals, liquid oral A6 IK9/00 (2006.01) dosage forms and other dosage forms. -

Steroid Abuse by School Age Children
epartment .D of .S Ju U s t ic U.S. Department of Justice e . n o i Drug Enforcement Administration t a r t is D in Office of Diversion Control r m ug d E t A nforcemen www.dea.gov www.DEAdiversion.usdoj.gov epartment .D of .S Ju U s t ic e . n o i t a r t is D in r m ug d E t A nforcemen Office of Diversion Control www.dea.gov STEROID ABUSE BY SCHOOL AGE CHILDREN nce viewed as a problem strictly associated with body builders, Ofitness “buffs,” and professional athletes, abuse of anabolic steroids by school age children has significantly increased over the past decade. The National Institute on Drug Abuse (NIDA) estimates that more than a half million 8th and 10th grade students are now using these dangerous drugs, and increasing numbers of high school seniors do not believe steroids are risky. Students are acquiring and taking anabolic steroids without any knowledge of the dangers associated with steroid abuse. The short- term adverse physical effects of anabolic steroid abuse are fairly well known. However, the long-term adverse physical effects of anabolic steroid abuse have not been studied, and as such, are not known. In addition, this type of abuse may result in harmful side-effects as well as serious injury and death. The abuser in most cases is unaware of these hidden dangers. Presented as a public service by: This guide will help you understand why steroids are being misused, Drug Enforcement Administration and how you can provide counseling and implement procedures to Office of Diversion Control educate our youth about the dangers of these drugs. -

(12) United States Patent (10) Patent No.: US 6,284,263 B1 Place (45) Date of Patent: Sep
USOO6284263B1 (12) United States Patent (10) Patent No.: US 6,284,263 B1 Place (45) Date of Patent: Sep. 4, 2001 (54) BUCCAL DRUG ADMINISTRATION IN THE 4,755,386 7/1988 Hsiao et al. TREATMENT OF FEMALE SEXUAL 4,764,378 8/1988 Keith et al.. DYSFUNCTION 4,877,774 10/1989 Pitha et al.. 5,135,752 8/1992 Snipes. 5,190,967 3/1993 Riley. (76) Inventor: Virgil A. Place, P.O. Box 44555-10 5,346,701 9/1994 Heiber et al. Ala Kahua, Kawaihae, HI (US) 96743 5,516,523 5/1996 Heiber et al. 5,543,154 8/1996 Rork et al. ........................ 424/133.1 (*) Notice: Subject to any disclaimer, the term of this 5,639,743 6/1997 Kaswan et al. patent is extended or adjusted under 35 6,180,682 1/2001 Place. U.S.C. 154(b) by 0 days. * cited by examiner (21) Appl. No.: 09/626,772 Primary Examiner Thurman K. Page ASSistant Examiner-Rachel M. Bennett (22) Filed: Jul. 27, 2000 (74) Attorney, Agent, or Firm-Dianne E. Reed; Reed & Related U.S. Application Data ASSciates (62) Division of application No. 09/237,713, filed on Jan. 26, (57) ABSTRACT 1999, now Pat. No. 6,117,446. A buccal dosage unit is provided for administering a com (51) Int. Cl. ............................. A61F 13/02; A61 K9/20; bination of Steroidal active agents to a female individual. A61K 47/30 The novel buccal drug delivery Systems may be used in (52) U.S. Cl. .......................... 424/435; 424/434; 424/464; female hormone replacement therapy, in female 514/772.3 contraception, to treat female Sexual dysfunction, and to treat or prevent a variety of conditions and disorders which (58) Field of Search .................................... -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9 -

The Combined Cardiac Effect of the Anabolic Steroid, Nandrolone And
ù1. v -¿. rlc) 77.- *n*hi.rnool oowol,ù*o ffi"/fu -lo *rn*(o fii'o fio¿o¿¿, /v&"ùún lonno **al cooaiæe';¿vfl"- oã. Benjamin D. Phillis, B.Sc. (Hons) Phatmacology Depattment of Clinical & Experimental Pharmacology Ftome Rd. , Medical School Noth Adelaide Univetsity ADEIAIDE SA 5OOO û.)r.'-*hr/7enveltîù Foremost, I would like to thank my two supervisors for the direction that they have given this ptojecr. To Rod, for his unfailing troubleshooting abiJity and to Jenny fot her advice and ability to add scientific rigour' Many thanks to Michael Adams for his technical assistance and especially fot performing the surgery for the ischaemia-reperfusion projects and for his willingness to work late nights and public holidays. Lastly I would like to thank my v¡ife for her extreme patience during the tumult of the last 5 years. Her love, suppoït, patience and undetstanding have been invaluable in this endeavout. Beniamin D. Phillis Octobet,2005 ADE,I-AIDE ii T*(¿t of Ao,t",tù DECI.ARATION I ACKNOWLEDGEMENTS il TABLE OF CONTENTS UI ABBREVIATIONS x ABSTRACT )ilr CÉIAPTER t-l Inttoduction 1-1 1.1 Background 1,-1, 1.2What ate anabolic stetoids? 7-1 1,3 General pharmacology of Anabolic steroids t-2 '1,-2 1.3.1 Genomic effects of anabolic steroids 1.3.2 Non-genomic effects of anabolic steroids 1-3 1.4 Clinical use of AS 1.-4 1.5 Patterns of AS abuse 1.-4 1.5.1 Steroid abuse by athletes 1.-+ 1.5.2 Stetoid abuse by sedentary teenagers r-6 1.5.3 Prevalence of abuse 1-6 1.5.4 Abuse ptevalence in Australia 1.-9 1.6 Cardiotoxicity of anabolic steroids r-9 1.6.1 Reduced cotonary flow 1.-1.1, 1,.6.2 Dtect myocatdial eff ects 1-1 5 1.6.3 Hypertension 1-21 1.7 Difficulties associated with anabolic steroid research 1.-24 1-25 1.8 The polydrug abuse Phenomenon 1.9 The pharmacology of cocaine 1-26 1.10 Pteparations 1-28 1-29 1.11 Metabolism lll 1-30 1. -

Interactions of Nandrolone and Psychostimulant Drugs on Central Monoaminergic Systems. National Institute for Health and Welfare (THL), Research 30
Sanna Kailanto Sanna Kailanto Interactions of Nandrolone Sanna Kailanto and Psychostimulant Drugs RESEARCH Interactions of Nandrolone and RESEARCH Psychostimulant Drugs on Central on Central Monoaminergic Monoaminergic Systems Systems Monoaminergic Systems Monoaminergic Central on Drugs Psychostimulant and Nandrolone of Interactions This study had four main aims. First, it aimed to explore the effects of nandrolone decanoate on dopaminergic and serotonergic activities in rat brains. Second, it set out to assess whether nandrolone pre-exposure modulates the acute neurochemical and behavioral effects of psychostimulant drugs in experimental animals. A third aim was to investigate if AAS-pretreatment-induced changes in brain reward circuitry are reversible. Finally, the study was also intended to evaluate the role of androgen receptors in nandrolone’s ability to modulate the dopaminergic and serotonergic effects of stimulants. The results of the study show that AAS pretreatment inhibits the reward- related neurochemical and behavioral effects of amphetamine, MDMA and cocaine in experimental animals. Given that LMA, stereotyped behavior and accumbal outflow of DA and 5-HT are all related to reward, this study suggests that nandrolone, at tested doses, significantly affects the rewarding properties of stimulant drugs. Furthermore, it seems that these effects could be long- lasting and that the ability of nandrolone to modulate reward-related effects of stimulants depends on AR or ER activation. .!7BC5<2"HIFILD! National Institute for Health and Welfare P.O. Box 30 (Mannerheimintie 166) FI-00271 Helsinki, Finland Telephone: +358 20 610 6000 30 ISBN 978-952-245-258-0 www.thl.fi 30 2010 30 Sanna Kailanto Interactions of Nandrolone and Psychostimulant Drugs on Central Monoaminergic Systems Academic dissertAtIoN To be presented with the permission of the Faculty of Biological and Environmental Sciences, University of Helsinki, for public examination in the Arppeanum auditorium, Helsinki University Museum, Snellmaninkatu 3, Helsinki, on April 29nd, at 12 o’clock noon. -

Pharmacology
STATE ESTABLISHMENT «DNIPROPETROVSK MEDICAL ACADEMY OF HEALTH MINISTRY OF UKRAINE» V.I. MAMCHUR, V.I. OPRYSHKO, А.А. NEFEDOV, A.E. LIEVYKH, E.V.KHOMIAK PHARMACOLOGY WORKBOOK FOR PRACTICAL CLASSES FOR FOREIGN STUDENTS STOMATOLOGY DEPARTMENT DNEPROPETROVSK - 2016 2 UDC: 378.180.6:61:615(075.5) Pharmacology. Workbook for practical classes for foreign stomatology students / V.Y. Mamchur, V.I. Opryshko, A.A. Nefedov. - Dnepropetrovsk, 2016. – 186 p. Reviewed by: N.I. Voloshchuk - MD, Professor of Pharmacology "Vinnitsa N.I. Pirogov National Medical University.‖ L.V. Savchenkova – Doctor of Medicine, Professor, Head of the Department of Clinical Pharmacology, State Establishment ―Lugansk state medical university‖ E.A. Podpletnyaya – Doctor of Pharmacy, Professor, Head of the Department of General and Clinical Pharmacy, State Establishment ―Dnipropetrovsk medical academy of Health Ministry of Ukraine‖ Approved and recommended for publication by the CMC of State Establishment ―Dnipropetrovsk medical academy of Health Ministry of Ukraine‖ (protocol №3 from 25.12.2012). The educational tutorial contains materials for practical classes and final module control on Pharmacology. The tutorial was prepared to improve self-learning of Pharmacology and optimization of practical classes. It contains questions for self-study for practical classes and final module control, prescription tasks, pharmacological terms that students must know in a particular topic, medical forms of main drugs, multiple choice questions (tests) for self- control, basic and additional references. This tutorial is also a student workbook that provides the entire scope of student’s work during Pharmacology course according to the credit-modular system. The tutorial was drawn up in accordance with the working program on Pharmacology approved by CMC of SE ―Dnipropetrovsk medical academy of Health Ministry of Ukraine‖ on the basis of the standard program on Pharmacology for stomatology students of III - IV levels of accreditation in the specialties Stomatology – 7.110105, Kiev 2011. -

Nandrolone Decanoate Inhibits Gluconeogenesis and Decreases Fasting Glucose in Wistar Male Rats
S P FRANKENFELD and others Nandrolone decanoate and 220:2 143–153 Research glucose metabolism Nandrolone decanoate inhibits gluconeogenesis and decreases fasting glucose in Wistar male rats Stephan Pinheiro Frankenfeld1, Leonardo Pires de Oliveira3, Daniele Lea˜o Ignacio3, Raquel Guimara˜es Coelho2, Mariana Nigro Mattos4, Andrea Claudia Freitas Ferreira2, Denise Pires Carvalho2 and Rodrigo Soares Fortunato1 1Laboratory of Molecular Radiobiology, Institute of Biophysics Carlos Chagas Filho, UFRJ, CCS, 2Laboratory of Correspondence Endocrine Physiology, Institute of Biophysics Carlos Chagas Filho, 3Laboratory of Exercise Biology, should be addressed School of Physical Education and Sports and 4Laboratory of Bioenergetics, Institute of Medical Biochemistry, to R S Fortunato Federal University of Rio de Janeiro, Avenida Carlos Chagas Filho, 373, Block G - Underground - Room G0-031, Email 21941-902 Rio de Janeiro, RJ, Brazil [email protected] Abstract The use of anabolic–androgenic steroids to improve physical performance or appearance has Key Words increased notably. The doses used are 10- to 100- fold higher than the therapeutic dose (TD), " glucose and this abuse can cause several side effects. Glucose metabolism is significantly affected by " anabolic–androgenic steroids anabolic–androgenic steroid abuse, but studies about glycemic regulation during fasting are " gluconeogenesis scarce. There are some evidences showing that testosterone can antagonize glucocorticoids " insulin action, which are crucial to glucose production during fasting. Thus, the aim of this study was to determine the impact of supraphysiological doses (SDs) of nandrolone decanoate Journal of Endocrinology (DECA) on rat glucose metabolism during fasting. Male Wistar rats were treated with i.m. injections of vehicle, a low TD (0.016 mg/100 g b.w.-TD group) or a high SD (1 mg/100 g b.w.-SD group) of DECA, once a week for 8 weeks. -

Norethisterone(BAN, Pinn)
Nandrolone/Norethisterone 2119 fluid retention or adverse effect on renal function or serum-lipids. Pharmacopoeias. In Eur. (see p.vii). Mex.: Evra; Neth.: Evra; Norw.: Evra; Philipp.: Evra; Pol.: Evra; Port.: In these reports, intramuscular doses of nandrolone decanoate Ph. Eur. 6.2 (Nomegestrol Acetate). A white or almost white Evra; Rus.: Evra (Евра); S.Afr.: Evra; Singapore: Evra; Spain: Evra; Swed.: have ranged from 100 mg once every 2 weeks4 up to 600 mg crystalline powder. Practically insoluble in water; soluble in al- Evra; Switz.: Evra; Thai.: Evra; UK: Evra; USA: Ortho Evra; Venez.: Evra. weekly,2 and treatment has generally been given for 12 to 24 cohol; freely soluble in acetone. Protect from light. weeks. Profile Norethandrolone (BAN, rINN) ⊗ 1. Gold J, et al. Safety and efficacy of nandrolone decanoate for Nomegestrol acetate is a progestogen structurally related to pro- treatment of wasting in patients with HIV infection. AIDS 1996 17α-Ethyl-17β-hydroxyestr-4-en-3-one; 17β-Hydroxy-19-nor- 10: 745–52. gesterone (p.2125) that has been used in the treatment of men- 2. Sattler FR, et al. Effects of pharmacological doses of nandrolone strual disorders and as the progestogen component of menopau- 17α-pregn-4-en-3-one; Noretandrolona; Noréthandrolone; decanoate and progressive resistance training in immunodefi- sal HRT (p.2071). Typical oral doses are 5 mg daily for 10 to 14 Norethandrolonum. cient patients infected with human immunodeficiency virus. J days of a 28-day cycle. A subdermal implant is under investiga- Норэтандролон Clin Endocrinol Metab 1999; 84: 1268–76. tion as a long-acting progestogen-only contraceptive. -

Interactions of Nandrolone and Psychostimulant Drugs on Central Monoaminergic Systems
Sanna Kailanto Sanna Kailanto Interactions of Nandrolone Sanna Kailanto and Psychostimulant Drugs RESEARCH Interactions of Nandrolone and RESEARCH Psychostimulant Drugs on Central on Central Monoaminergic Monoaminergic Systems Systems Monoaminergic Systems Monoaminergic Central on Drugs Psychostimulant and Nandrolone of Interactions This study had four main aims. First, it aimed to explore the effects of nandrolone decanoate on dopaminergic and serotonergic activities in rat brains. Second, it set out to assess whether nandrolone pre-exposure modulates the acute neurochemical and behavioral effects of psychostimulant drugs in experimental animals. A third aim was to investigate if AAS-pretreatment-induced changes in brain reward circuitry are reversible. Finally, the study was also intended to evaluate the role of androgen receptors in nandrolone’s ability to modulate the dopaminergic and serotonergic effects of stimulants. The results of the study show that AAS pretreatment inhibits the reward- related neurochemical and behavioral effects of amphetamine, MDMA and cocaine in experimental animals. Given that LMA, stereotyped behavior and accumbal outflow of DA and 5-HT are all related to reward, this study suggests that nandrolone, at tested doses, significantly affects the rewarding properties of stimulant drugs. Furthermore, it seems that these effects could be long- lasting and that the ability of nandrolone to modulate reward-related effects of stimulants depends on AR or ER activation. .!7BC5<2"HIFILD! National Institute for Health and Welfare P.O. Box 30 (Mannerheimintie 166) FI-00271 Helsinki, Finland Telephone: +358 20 610 6000 30 ISBN 978-952-245-258-0 www.thl.fi 30 2010 30 Sanna Kailanto Interactions of Nandrolone and Psychostimulant Drugs on Central Monoaminergic Systems Academic disSertAtIoN To be presented with the permission of the Faculty of Biological and Environmental Sciences, University of Helsinki, for public examination in the Arppeanum auditorium, Helsinki University Museum, Snellmaninkatu 3, Helsinki, on April 29nd, at 12 o’clock noon. -
EXECUTIVE SUMMARY Steroids
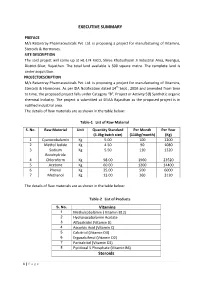
EXECUTIVE SUMMARY PREFACE M/s Ratantray Pharmaceuticals Pvt. Ltd. is proposing a project for manufacturing of Vitamins, Steroids & Hormones. SITE DESCRIPTION The said project will come up at H1-174 RIICO, Shree Khatushyam Ji Industrial Area, Reengus, District-Sikar, Rajasthan. The total land available is 500 square metre. The complete land is under acquisition. PROJECTDESCRIPTION M/s Ratantray Pharmaceuticals Pvt. Ltd. is proposing a project for manufacturing of Vitamins, Steroids & Hormones. As per EIA Notification dated 14th Sept., 2006 and amended from time to time, the proposed project falls under Category “B”, Project or Activity 5(f) Synthetic organic chemical Industry. The project is submitted at SEIAA Rajasthan as the proposed project is in notified industrial area. The details of Raw materials are as shown in the table below: Table-1 List of Raw Material S. No. Raw Material Unit Quantity Standard Per Month Per Year (5.0kg batch size) (110kg/month) (Kg) 1 Cyanocobalamin Kg 5.00 100 1200 2 Methyl Iodide Kg 4.50 90 1080 3 Sodium Kg 5.50 110 1320 Borohydride 4 Chloroform Kg 98.00 1960 23520 5 Acetone Kg 60.00 1200 14400 6 Phenol Kg 25.00 500 6000 7 Methanol Kg 13.00 260 3120 The details of Raw materials are as shown in the table below: Table-2 List of Products S. No. Vitamins 1 Methylcobalamin ( Vitamin B12) 2 Hydroxocobalamin Acetate 3 Alfacalcidol (Vitamin D) 4 Ascorbic Acid (Vitamin C) 5 Calcitriol (Vitamin D3) 6 Ergocalciferol (Vitamin D2) 7 Paricalcitol (Vitamin D2) 8 Pyridoxal 5 Phosphate (Vitamin B6) Steroids 1 | Page 1 Beclomethasone