Proquest Dissertations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

England Soccer
Wochenangebot Gültig ab 27.08.2021 bis 03.09.2021 Soccer - England Premier League 1 X 2 1X 12 2X HC 1 X 2 T - + 42B Sa 12:30 Manchester City FC : Arsenal FC 1,25 6,25 11,15 1,01 1,09 3,90 0:1 1,70 4,00 3,85 2.5 2,40 1,55 434 Sa 15:00 Norwich City FC : Leicester City FC 4,05 3,75 1,85 1,90 1,24 1,21 1:0 1,85 4,00 3,15 2.5 2,10 1,70 435 Sa 15:00 Aston Villa : Brentford FC 2,00 3,50 3,80 1,23 1,27 1,80 0:1 3,70 3,90 1,75 2.5 1,80 2,00 433 Sa 15:00 Brighton & Hove : Everton FC 2,30 3,20 3,30 1,30 1,30 1,60 0:1 4,80 4,05 1,55 2.5 1,60 2,30 Albion FC 436 Sa 15:00 Newcastle United : Southampton FC 2,45 3,45 2,85 1,40 1,28 1,50 0:1 4,85 4,50 1,50 2.5 2,00 1,80 438 Sa 15:00 West Ham United : Crystal Palace FC 1,50 4,40 6,30 1,09 1,18 2,50 0:1 2,35 3,65 2,45 2.5 1,95 1,80 39C Sa 17:30 Liverpool FC : Chelsea FC 2,45 3,30 2,95 1,35 1,30 1,50 0:1 5,00 4,35 1,50 2.5 1,80 1,95 368 So 14:00 Burnley FC : Leeds United 3,10 3,50 2,30 1,60 1,28 1,35 1:0 1,55 4,45 4,25 2.5 2,15 1,70 393 So 14:00 Tottenham Hotspur : Watford FC 1,45 4,65 7,20 1,07 1,17 2,75 0:1 2,20 3,65 2,70 2.5 1,95 1,80 33B So 16:30 Wolverhampton : Manchester United 4,55 3,65 1,80 1,95 1,25 1,17 1:0 1,95 3,80 3,10 2.5 1,90 1,90 Wanderers FC FC Soccer - England Championship 1 X 2 1X 12 2X HC 1 X 2 T - + 4D7 Sa 12:30 Cardiff City : Bristol City 1,90 3,50 4,25 1,19 1,27 1,85 0:1 3,45 3,80 1,85 2.5 1,75 2,10 45A Sa 12:30 Derby County : Nottingham Forest 2,50 3,00 3,15 1,35 1,35 1,50 0:1 5,65 4,15 1,45 1.5 2,60 1,50 4C9 Sa 15:00 Huddersfield Town : Reading FC 2,35 3,30 3,10 1,35 1,30 1,55 0:1 -

Damon Simmons WR 6-3 185 R-So
BARKER-LANE STADIUM • 13th ranked stadium nationally in FCS BRIAN HUDSON • Calgary Stampeders (CFL) signee • 8 single-season offensive records KURT ODOM • 1st Campbell Football All-American 21 OFFENSIVE TEAM RECORDS • 2,800 rushing yards • 2,436 passing yards • 5,236 total offense 5 DEFENSIVE TEAM RECORDS • 27 sacks 3 SPECIAL TEAMS RECORDS • 3 special teams touchdowns 2014 CAMPBELL UNIVERSITY FOOTBALL #GOCAMELS GENERAL TEAM INFORMATION School ......................................................................Campbell University 2013 Overall Record ..........................................................................3-9 Location ...................................................................... Buies Creek, N.C. PFL Record .......................................................................................2-6 Founded ........................................................................ January 5, 1887 Home Record ....................................................................................1-5 Enrollment ...............................6834 (all campuses), 4663 (main campus) Road Record .....................................................................................2-4 President ..................................................................Dr. Jerry M. Wallace PFL Finish .........................................................................................8th Alma Mater ................................................................... East Carolina ‘56 Off. Starters Returning/Lost................................................................8/3 -

Lost Angels: Psychoanalysis and Cinema/Vicky Lebeau
Lost Angels In Lost Angels, Vicky Lebeau re-reads Freudian theories of femininity to develop a remarkable contribution to spectatorship theory. Lebeau discusses Freud’s distinctive preoccupations with female fantasy and femininity—from his studies on hysteria and the ‘family romance’ at the origins of psychoanalysis to the analysis of mass psychology in the 1920s and 1930s. Lost Angels exposes how Freud’s accounting of femininity is intimately tied to his changing representation of the paternal, and explores his ensuing differentiation between masculine and feminine fantasy through critical and feminist theories of spectatorship and cinema. Discussing three popular ‘youth’ films of the 1980s—John Hughes’s Ferris Bueller’s Day Off, Francis Ford Coppola’s Rumble Fish and Tim Hunter’s River’s Edge—Lebeau works through issues of sexual difference and social identification and creates a dialogue between feminism, psychoanalysis and the critical theory of the Frankfurt school. Intervening in current debates on femininity, fantasy and identification, Lebeau suggests that, for Freud, femininity is always both a sexed and a social category which cannot be understood outside of its relation to the father. Lost Angels is a ground-breaking addition to current feminist film theory and essential reading for all students of film, gender and cultural studies. Vicky Lebeau is Lecturer in English in the School of Cultural and Community Studies at the University of Sussex. Frontispiece: production still from Robert Wiene’s The Cabinet of Dr. Caligari (1919). Courtesy BFI. Lost Angels Psychoanalysis and cinema Vicky Lebeau London and New York First published 1995 by Routledge 11 New Fetter Lane, London EC4P 4EE This edition published in the Taylor & Francis e-Library, 2005. -

Pli Volume 13
Contents Foucault: Madness/Sexuality/Biopolitics First Preface to Histoire de la folie à l’âge classique (1961) MICHEL FOUCAULT 1 Preface to the Italian edition of La volonté de savoir MICHEL FOUCAULT 11 Astride a Low Wall: Notes on Philosophy and Madness PIER ALDO ROVATTI 13 Lucid Unreason: Artaud and Foucault LORENZO CHIESA 26 Logics of Delirium REMO BODEI 66 Words, Desires and Ideas: Freud, Foucault and the Hermaphroditic Roots of Bisexuality SHARON COWAN & STUART ELDEN 79 From Biopower to Biopolitics MAURIZIO LAZZARATO 100 Toward a Conception of Racism without Race: Foucault and Contemporary Biopolitics WARREN MONTAG 113 A Fugitive Thread: The Production of Subjectivity in Marx JASON READ 126 Varia Teleosemantics and the Genesis of Norms: Co-opting Brandom TIM SCHROEDER 145 When Latour meets Nietzsche Around the Concept of Individuation JONATHAN PHILIPPE 162 Alain Badiou’s Theory of the Subject: The Re-Commencement of Dialectical Materialism (Part II) BRUNO BOSTEELS 173 Towards a Material Imaginary: Bataille, Nonlogical Difference, and the Language of Base Materialism F. SCOTT SCRIBNER 209 Reviews Peter Abelard’s Stoic Ethics JOHN SELLARS 222 What’s a Brain Supposed to Do? MICHAEL FERRER 229 Pli 13 (2002), 1-10. First Preface to Histoire de la folie à l’âge classique (1961)1 MICHEL FOUCAULT Pascal: “Men are so necessarily mad, that not to be mad would amount to another form of madness.” And this other text, by Dostoyevsky, from his Diary of a Writer: “It is not by confining one’s neighbour that one is convinced of one’s good sense.” We have yet to write the history of that other turn of madness, whereby men, in the gesture of sovereign reason that confines their neighbour, communicate and recognise each other through the merciless language of non-madness; to recover the moment of this conjuration, before madness was definitively established in the realm of truth, before it was revived by the lyricism of protest. -
![Download Document [Optimized]](https://docslib.b-cdn.net/cover/9921/download-document-optimized-5339921.webp)
Download Document [Optimized]
PREMIUM PORTABLE MIXER-RECORDER USER GUIDE Legal Notices Manual Conventions Product specifications and features are subject to change without prior notification. SSYMBOLYMBOL DESCRIPTION This symbol is used to show the order in which you select menu © Copyright 2020 Sound Devices, LLC. All rights reserved. > commands and sub-options, such as: Main Menu > Outputs This product is subject to the terms and conditions of a indicates you press the Menu button for the Main Menu, then software license agreement provided with the product, and scroll to and select Outputs by pushing the Knob. may be used in accordance with the license agreement. [ ] This symbol is used to convey selectable menu items. This document is protected under copyright law. An authorized * licensee of this product may reproduce this publication for the This symbol is used to convey factory default settings. licensee’s own personal use. This document may not be reproduced A plus sign is used to show button or keystroke combinations. For or distributed, in whole or in part, for commercial purposes, such instance, Ctrl+V means to hold the Control key down and press as selling copies or providing educational services or support. the V key simultaneously. This also applies to other controls, This document is supplied as a technical guide. Special care + such as switches and knobs. For instance, MIC+HP turn means has been taken in preparing the information for publication; to slide and hold the MIC/TONE switch left while turning the Headphone (HP) knob. METERS+SELECT means to hold the however, since product specifications are subject to change, METERS button down as you press the SELECT knob. -

FB Mediaguide 2018.Pdf
2018 CAMPBELL FOOTBALL TABLE OF CONTENTS Table of Contents .................................................................................1 2018 Camels (listed alphabetically) Quick Facts, 2018 Schedule ...................................................................2 Returners 2017 Results, 2017 PFL/BSC Final Standings, Pronunciation Guide Adams-Barr .................................................................................. 32-33 Blockmon-Burress ......................................................................... 34-35 2018 Season Preview Charles-Eason-Riddle ..................................................................... 36-37 Numerical Roster ...............................................................................3-4 Ferguson-Harper ........................................................................... 38-39 Alphabetical Roster ............................................................................5-6 Hartshorn-Holmes ......................................................................... 40-41 Roster Breakdown .............................................................................6-7 Howard-King ................................................................................. 42-43 Preseason Two-Deep ............................................................................8 Layden-McNeely ............................................................................ 44-45 Mike Minter preseason Q&A .............................................................9-10 Miller-Price ................................................................................... -

Official League Stats 2018 Season Index
OFFICIAL LEAGUE STATS 2018 SEASON INDEX 01 | CHAMPIONSHIP STATS AND RESULTS ......................................... 3 2018 PFL WORLD CHAMPIONS .......................................................................... 3 2018 PFL CHAMPIONSHIP RESULTS................................................................... 3 2018 PFL CHAMPIONSHIP SUPERLATIVES .......................................................... 4 SEEDS - CHAMPIONSHIP APPEARANCES ........................................................... 4 SEEDS - CHAMPIONSHIPS WON ........................................................................ 4 02 | LEAGUE STATS ......................................................................... 5 STOPPAGE PERCENTAGE ................................................................................... 5 STOPPAGE BREAKDOWN ................................................................................... 5 FIGHTING TWICE IN ONE NIGHT ........................................................................ 5 FIGHT SCHEDULING - SUCCESS RATE ................................................................ 5 NATIONS REPRESENTED ................................................................................... 5 03 | CAGENOMICS .......................................................................... 6 SINGLE FIGHT LEADERS ................................................................................... 6 SEASON LEADERS ........................................................................................... 9 04 | OFFICIAL LEAGUE RESULTS -

Premium Portable Mixer-Recorder
PREMIUM PORTABLE MIXER-RECORDER USER GUIDE Legal Notices Manual Conventions Product specifications and features are subject to change without prior notification. SYMBOL DESCRIPTION This symbol is used to show the order in which you select menu Copyright© 2019 Sound Devices, LLC. All rights reserved. commands and sub-options, such as: Main Menu > Outputs > This product is subject to the terms and conditions of a indicates you press the Menu button for the Main Menu, then software license agreement provided with the product, and scroll to and select Outputs by pushing the Encoder. may be used in accordance with the license agreement. [ ] This symbol is used to convey selectable menu items. This document is protected under copyright law. An authorized licensee of this product may reproduce this publication for the * This symbol is used to convey factory default settings. licensee’s own personal use. This document may not be reproduced A plus sign is used to show button or keystroke combinations. For or distributed, in whole or in part, for commercial purposes, such instance, Ctrl+V means to hold the Control key down and press as selling copies or providing educational services or support. the V key simultaneously. This also applies to other controls, such This document is supplied as a technical guide. Special care + as switches and encoders. For instance, MIC+HP turn means has been taken in preparing the information for publication; to slide and hold the MIC/TONE switch left while turning the Headphone (HP) encoder. METERS+SELECT means to hold the however, since product specifications are subject to change, METERS button down as you press the SELECT encoder. -

Lustre Feature Test Plan for Progressive File Layout
Lustre Feature Test Plan for Progressive File Layout Revision 0.3 April 6, 2017 Revision History The following is a chronological history of changes made to this document. Revision Date Reason for change Author 0.1 March 2, Initial Version James Nunez 2017 0.2 April 3, 2017 Added list of tests in sanity-pfl, James Nunez added performance tests and updated requirements to test suite/test number mapping 0.3 April 6, 2017 Corrected ‘lfs getstripe’ flag James Nunez descriptions to reflect when a flag argument was optional or not. Removed –component-id flag from ‘lfs find’ Table of Contents Revision History ................................................................................................ 2 Introduction ...................................................................................................... 5 Documentation .................................................................................................. 6 Feature Installation and Set-up ....................................................................... 6 Regression Tests ................................................................................................ 6 Manual Testing ................................................................................................. 6 Interoperability ................................................................................................. 6 Performance Testing ......................................................................................... 7 User Interface .................................................................................................. -
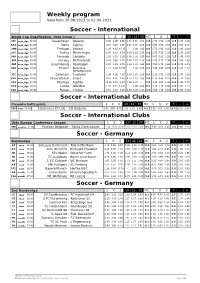
Weekly Program Soccer
Weekly program Valid from 26.08.2021 to 02.09.2021 Soccer - International World Cup Qualification, Uefa Group J 1 X 2 1X 12 2X HC 1 X 2 T - + Kazakhstan : Ukraine 8.05 4.45 1.45 2.70 1.16 1.03 1:0 2.70 3.50 2.25 2.5 1.70 1.95 16:00 ﭼﻬﺎرﺷﻨﺒﻪ 36C Malta : Cyprus 4.70 3.25 1.95 1.80 1.30 1.15 1:0 1.80 3.80 3.55 2.5 1.55 2.15 20:45 ﭼﻬﺎرﺷﻨﺒﻪ 3A A Portugal : Ireland 1.25 6.15 14.35 - 1.08 4.05 0:1 1.75 3.65 4.05 2.5 1.85 1.80 20:45 ﭼﻬﺎرﺷﻨﺒﻪ 2DB Turkey : Montenegro 1.60 4.15 6.30 1.08 1.19 2.35 0:1 2.55 3.55 2.35 2.5 1.70 1.95 20:45 ﭼﻬﺎرﺷﻨﺒﻪ 367 Slovenia : Slovakia 2.35 3.25 3.35 1.28 1.30 1.55 0:1 5.05 3.95 1.55 2.5 1.45 2.45 20:45 ﭼﻬﺎرﺷﻨﺒﻪ 36A Norway : Netherlands 5.05 3.85 1.75 2.05 1.22 1.13 1:0 2.05 3.75 2.90 2.5 1.80 1.85 20:45 ﭼﻬﺎرﺷﻨﺒﻪ 35F Luxembourg : Azerbaijan 2.20 3.25 3.75 1.23 1.29 1.65 0:1 4.55 3.75 1.65 1.5 2.45 1.45 20:45 ﭼﻬﺎرﺷﻨﺒﻪ 2BE France : Bosnia & 1.21 6.90 16.95 - 1.06 4.60 0:1 1.60 3.85 4.60 2.5 1.95 1.70 20:45 ﭼﻬﺎرﺷﻨﺒﻪ 303 Herzegovina Denmark : Scotland 1.45 4.60 7.55 1.04 1.15 2.65 0:1 2.25 3.55 2.65 2.5 1.75 1.90 20:45 ﭼﻬﺎرﺷﻨﺒﻪ 31C Faroe Islands : Israel 8.05 4.65 1.45 2.75 1.15 1.03 1:0 2.75 3.45 2.25 2.5 1.70 1.95 20:45 ﭼﻬﺎرﺷﻨﺒﻪ 31A Moldova : Austria 15.25 6.45 1.23 4.25 1.07 - 1:0 4.25 3.80 1.65 2.5 1.90 1.75 20:45 ﭼﻬﺎرﺷﻨﺒﻪ 319 Latvia : Gibraltar 1.26 6.15 14.05 - 1.08 4.00 0:1 1.70 3.70 4.00 2.5 1.85 1.75 20:45 ﭼﻬﺎرﺷﻨﺒﻪ 3A 9 Russia : Croatia 3.15 3.40 2.40 1.55 1.28 1.30 1:0 1.55 4.15 4.80 2.5 1.65 2.05 20:45 ﭼﻬﺎرﺷﻨﺒﻪ 28A Soccer - International Clubs Freundschaftsspiele 1 X 2 1X 12 2X HC 1 X 2 T - + Salamanca CF Uds : CD Guijuelo 1.90 3.80 4.05 1.20 1.22 1.85 0:1 3.25 4.05 1.85 2.5 2.05 1.65 20:00 ﺷﻨﺒﻪ C20 Soccer - International Clubs Uefa Europa Conference League 1 X 2 1X 12 2X HC 1 X 2 T - + Partizan Belgrade : Santa Clara Azores 1.75 3.75 5.25 1.11 1.23 2.05 0:1 3.00 3.55 2.05 2.5 1.60 2.10 21:00 ﭘﻨﺠﺸﻨﺒﻪ 3E8 Soccer - Germany Bundesliga 1 X 2 1X 12 2X HC 1 X 2 T - + Borussia Dortmund : TSG Hoffenheim 1.45 5.45 6.00 1.08 1.10 2.70 0:1 2.00 4.30 2.70 3.5 1.90 1.75 20:30 ﺟﻤﻌﻪ A F Arm. -

Wallace, Mason Sledge Jarrett Ozimek, Jim Malone
2017 CAMPBELL FOOTBALL TABLE OF CONTENTS Table of Contents .................................................................................1 2017 Camels (listed alphabetically) Quick Facts, 2017 Schedule ...................................................................2 Returners 2016 Results, 2016 PFL Final Standings, Pronunciation Guide Ashaye-Dixon ................................................................................ 32-35 Dobbins-Kelshaw ........................................................................... 36-39 2017 Season Preview Kemp-Miller .................................................................................. 40-43 Numerical Roster ...............................................................................3-4 Mitchell-Slade ............................................................................... 44-47 Alphabetical Roster ............................................................................5-6 Smith-Wooten ............................................................................... 48-50 Roster Breakdown .............................................................................6-7 Newcomers Preseason Two-Deep ............................................................................8 Anderson-Haywood ....................................................................... 51-53 Mike Minter preseason Q&A .............................................................9-10 Howard-Rivens .............................................................................. 54-55 Preseason