Crohn's Disease – Infliximab
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BAFF-Neutralizing Interaction of Belimumab Related to Its Therapeutic Efficacy for Treating Systemic Lupus Erythematosus
ARTICLE DOI: 10.1038/s41467-018-03620-2 OPEN BAFF-neutralizing interaction of belimumab related to its therapeutic efficacy for treating systemic lupus erythematosus Woori Shin1, Hyun Tae Lee1, Heejin Lim1, Sang Hyung Lee1, Ji Young Son1, Jee Un Lee1, Ki-Young Yoo1, Seong Eon Ryu2, Jaejun Rhie1, Ju Yeon Lee1 & Yong-Seok Heo1 1234567890():,; BAFF, a member of the TNF superfamily, has been recognized as a good target for auto- immune diseases. Belimumab, an anti-BAFF monoclonal antibody, was approved by the FDA for use in treating systemic lupus erythematosus. However, the molecular basis of BAFF neutralization by belimumab remains unclear. Here our crystal structure of the BAFF–belimumab Fab complex shows the precise epitope and the BAFF-neutralizing mechanism of belimumab, and demonstrates that the therapeutic activity of belimumab involves not only antagonizing the BAFF–receptor interaction, but also disrupting the for- mation of the more active BAFF 60-mer to favor the induction of the less active BAFF trimer through interaction with the flap region of BAFF. In addition, the belimumab HCDR3 loop mimics the DxL(V/L) motif of BAFF receptors, thereby binding to BAFF in a similar manner as endogenous BAFF receptors. Our data thus provides insights for the design of new drugs targeting BAFF for the treatment of autoimmune diseases. 1 Department of Chemistry, Konkuk University, 120 Neungdong-ro, Gwangjin-gu, Seoul 05029, Republic of Korea. 2 Department of Bio Engineering, Hanyang University, 222 Wangsimni-ro, Seongdong-gu, Seoul 04763, Republic of Korea. These authors contributed equally: Woori Shin, Hyun Tae Lee, Heejin Lim, Sang Hyung Lee. -

Old and New Challenges in Uveitis Associated with Behçet's Disease
Journal of Clinical Medicine Review Old and New Challenges in Uveitis Associated with Behçet’s Disease Julie Gueudry 1,* , Mathilde Leclercq 2, David Saadoun 3,4,5 and Bahram Bodaghi 6 1 Department of Ophthalmology, Hôpital Charles Nicolle, F-76000 Rouen, France 2 Department of Internal Medicine, Hôpital Charles Nicolle, F-76000 Rouen, France; [email protected] 3 Department of Internal Medicine and Clinical Immunology, AP-HP, Centre National de Références Maladies Autoimmunes et Systémiques Rares et Maladies Autoinflammatoires Rares, Groupe Hospitalier Pitié-Salpêtrière, F-75013 Paris, France; [email protected] 4 Sorbonne Universités, UPMC Univ Paris 06, INSERM, UMR S 959, Immunology-Immunopathology-Immunotherapy (I3), F-75005 Paris, France 5 Biotherapy (CIC-BTi), Hôpital Pitié-Salpêtrière, AP-HP, F-75651 Paris, France 6 Department of Ophthalmology, IHU FOReSIGHT, Sorbonne-AP-HP, Groupe Hospitalier Pitié-Salpêtrière, F-75013 Paris, France; [email protected] * Correspondence: [email protected]; Tel.: +33-2-32-88-80-57 Abstract: Behçet’s disease (BD) is a systemic vasculitis disease of unknown origin occurring in young people, which can be venous, arterial or both, classically occlusive. Ocular involvement is particularly frequent and severe; vascular occlusion secondary to retinal vasculitis may lead to rapid and severe loss of vision. Biologics have transformed the management of intraocular inflammation. However, the diagnosis of BD is still a major challenge. In the absence of a reliable biological marker, diagnosis is based on clinical diagnostic criteria and may be delayed after the appearance of the onset sign. However, therapeutic management of BD needs to be introduced early in order to control inflammation, to preserve visual function and to limit irreversible structural damage. -

Xolair/Omalizumab
Therapeutic/Humanized Antibodies ELISA Kits ‘Humanized antibodies” are ‘Animal Antibodies’ that have been modified by recombinant DNA-technology to reduce the overall content of the animal- portion of immunoglobulin so as to increase acceptance by humans or minimize ‘rejection’. By analogy, if the hands of a mouse is actually responsible for grabbing things then ‘humanized-mouse’ will only contain the ‘mouse hands’ and the rest of the body will be human. Since the size of the IgGs is similar in mouse and human, the size of the native mouse IgG and the ‘humanized IgG’ does not significantly change. Antigen recognition property of an antibody actually resides in small portion of the IgG molecule called the ‘antigen binding site or Fab’. Therefore, humanized antibodies contain the minimal portion from the Fab or the epitopes necessary for antigen binding. The process of "humanization of antibodies" is usually applied to animal (mouse) derived monoclonal antibodies for therapeutic use in humans (for example, antibodies developed as anti-cancer drugs). Xolair (Anti-IgE) is an example of humanized IgG. The portion of the mouse IgG that remains in the ‘humanized IgG’ may be recognized as foreign by humans and may result into the generation of “Human Anti-Drug Antibodies (HADA). The presence of anti-Drug antibody (e.g., Human Anti-Rituximab IgG) that may limit the long-term usage the humanized antibody (Rituximab). Not all monoclonal antibodies designed for human therapeutic use need be humanized since many therapies are short-term. The International Nonproprietary Names of humanized antibodies end in “-zumab”, as in “omalizumab”. Humanized antibodies are distinct from chimeric or fusion antibodies. -

Exploring the Association Between Monoclonal Antibodies and Depression and Suicidal Ideation and Behavior: a Vigibase Study
Drug Safety https://doi.org/10.1007/s40264-018-00789-9 ORIGINAL RESEARCH ARTICLE Exploring the Association between Monoclonal Antibodies and Depression and Suicidal Ideation and Behavior: A VigiBase Study Lotte A. Minnema1,2 · Thijs J. Giezen2,3 · Patrick C. Souverein1 · Toine C. G. Egberts1,4 · Hubert G. M. Leufkens1 · Helga Gardarsdottir1,4,5 © The Author(s) 2019 Abstract Introduction Several monoclonal antibodies (mAbs) have been linked to neuropsychiatric adverse efects in patients, includ- ing depression and suicidal ideation and behavior. Objective The aim of this study was to quantify and characterize spontaneously reported adverse drug reactions (ADRs) of depression and suicidal ideation and behavior related to mAb users, and to explore a possible association with their mecha- nism of action. Methods We included mAb ADRs that were reported in VigiBase, and identifed those related to depression and suicidal ideation and behavior. Reporting odds ratios (RORs) were estimated for each mAb (bevacizumab as the reference) and according to their infuence on the immune system (not directly targeting [reference], stimulating, or suppressing). Those suppressing the immune system were further divided into their intended indication (auto-immune diseases, cancer). Results Overall, 2,924,319 ADRs for 44 mAbs were included; 9455 ADRs were related to depression and 1770 were related to suicidal ideation and behavior. The association was strongest for natalizumab and belimumab, both for depression (ROR 5.7, 95% confdence interval [CI] 5.0–6.4; and ROR 5.1, 95% CI 4.2–6.2) and suicidal ideation and behavior (ROR 12.0, 95% CI 7.9–18.3; and ROR 20.2, 95% CI 12.4–33.0). -

Infliximab, Adalimumab and Golimumab for Treating Moderately
HEALTH TECHNOLOGY ASSESSMENT VOLUME 20 ISSUE 39 MAY 2016 ISSN 1366-5278 Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional therapy (including a review of TA140 and TA262): clinical effectiveness systematic review and economic model Rachel Archer, Paul Tappenden, Shijie Ren, Marrissa Martyn-St James, Rebecca Harvey, Hasan Basarir, John Stevens, Christopher Carroll, Anna Cantrell, Alan Lobo and Sami Hoque DOI 10.3310/hta20390 Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional therapy (including a review of TA140 and TA262): clinical effectiveness systematic review and economic model Rachel Archer,1* Paul Tappenden,1 Shijie Ren,1 Marrissa Martyn-St James,1 Rebecca Harvey,1 Hasan Basarir,1 John Stevens,1 Christopher Carroll,1 Anna Cantrell,1 Alan Lobo2 and Sami Hoque3 1Health Economics and Decision Science, School of Health and Related Research (ScHARR), University of Sheffield, Sheffield, UK 2Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK 3Barts Health NHS Trust, London, UK *Corresponding author Declared competing interests of authors: none Published May 2016 DOI: 10.3310/hta20390 This report should be referenced as follows: Archer R, Tappenden P, Ren S, Martyn-St James M, Harvey R, Basarir H, et al. Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional therapy (including a review of TA140 and TA262): clinical effectiveness systematic review and economic model. Health Technol Assess 2016;20(39). Health Technology Assessment is indexed and abstracted in Index Medicus/MEDLINE, Excerpta Medica/EMBASE, Science Citation Index Expanded (SciSearch®) and Current Contents®/ Clinical Medicine. -

Benlysta® (Belimumab)
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2021 P 1227-6 Program Prior Authorization/Notification Medication Benlysta® (belimumab)* *This program applies to the subcutaneous formulation of belimumab P&T Approval Date 9/2017, 9/2018, 9/2019, 9/2020, 2/2021, 7/2021 Effective Date 10/1/2021; Oxford only: 10/1/2021 1. Background: Benlysta® is a B-lymphocyte stimulator (BLyS)-specific inhibitor indicated for the treatment of patients aged 5 years and older with active, autoantibody-positive, systemic lupus erythematosus (SLE) who are receiving standard therapy and adult patients with active lupus nephritis who are receiving standard therapy. Limitations of Use: The efficacy of Benlysta has not been evaluated in patients with severe active central nervous system lupus. Benlysta has not been studied in combination with other biologics. Use of Benlysta is not recommended in these situations. 2. Coverage Criteria: A. Systemic Lupus Erythematosus 1. Initial Authorization a. Benlysta will be approved based on all of the following criteria: (1) Diagnosis of systemic lupus erythematosus -AND- (2) Laboratory testing has documented the presence of autoantibodies [e.g., ANA, Anti-dsDNA, Anti-Sm, Anti-Ro/SSA, Anti-La/SSB] -AND- (3) Patient is currently receiving standard immunosuppressive therapy [e.g., hydroxychloroquine, chloroquine, prednisone, azathioprine, methotrexate] -AND- (4) Patient does not have severe active central nervous system lupus -AND- (5) Patient is not receiving Benlysta in combination with either of the following: (a) Biologic DMARD [e.g., Enbrel (etanercept), Humira (adalimumab), Cimzia (certolizumab), Kineret (anakinra)] © 2021 UnitedHealthcare Services, Inc. 1 (b) Lupkynis (voclosporin) Authorization will be issued for 12 months. -

Cutaneous Adverse Effects of Biologic Medications
REVIEW CME MOC Selena R. Pasadyn, BA Daniel Knabel, MD Anthony P. Fernandez, MD, PhD Christine B. Warren, MD, MS Cleveland Clinic Lerner College Department of Pathology Co-Medical Director of Continuing Medical Education; Department of Dermatology, Cleveland Clinic; of Medicine of Case Western and Department of Dermatology, W.D. Steck Chair of Clinical Dermatology; Director of Clinical Assistant Professor, Cleveland Clinic Reserve University, Cleveland, OH Cleveland Clinic Medical and Inpatient Dermatology; Departments of Lerner College of Medicine of Case Western Dermatology and Pathology, Cleveland Clinic; Assistant Reserve University, Cleveland, OH Clinical Professor, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, OH Cutaneous adverse effects of biologic medications ABSTRACT iologic therapy encompasses an expo- B nentially expanding arena of medicine. Biologic therapies have become widely used but often As the name implies, biologic therapies are de- cause cutaneous adverse effects. The authors discuss the rived from living organisms and consist largely cutaneous adverse effects of tumor necrosis factor (TNF) of proteins, sugars, and nucleic acids. A clas- alpha inhibitors, epidermal growth factor receptor (EGFR) sic example of an early biologic medication is inhibitors, small-molecule tyrosine kinase inhibitors insulin. These therapies have revolutionized (TKIs), and cell surface-targeted monoclonal antibodies, medicine and offer targeted therapy for an including how to manage these reactions -

Neuromyelitis Optica Spectrum Disorder
© Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 | Fax 503-947-2596 Drug Class Review with New Drug Evaluation: Biologics for Autoimmune Disorders-Neuromyelitis Optica Spectrum Disorder Date of Review: April 2021 Date of Last Review: n/a Dates of Literature Search: 1/1/1996 – 1/20/2021 Generic Name: Brand Name (Manufacturer): Eculizumab Soliris® (Alexion Pharmaceuticals) Inebilizumab-cdon Uplizna™ (Viela Bio) Satralizumab-mwge Enspryng™ (Genentech/Roche) Dossiers Received: Yes Current Status of PDL Class: See Appendix 1. Purpose for Class Update: To define place in therapy for 3 immunosuppressive agents, eculizumab, inebilizumab-cdon, and satralizumab-mwge, recently approved by the Food and Drug Administration (FDA) for the treatment adults with neuromyelitis optica spectrum disorder (NMOSD). Research Questions: 1. What is the effectiveness of eculizumab, inebilizumab, and satralizumab in reducing time to relapse in adult patients with NMOSD who are anti-aquaporin-4 (AQP4) antibody positive? 2. What are the harms of eculizumab, inebilizumab-cdon and satralizumab in adults with NMOSD? 3. Is there comparative evidence that eculizumab, inebilizumab, and satralizumab differ in efficacy or harms for management of NMOSD? 4. Are there certain sub-populations (based on age, gender, ethnicity, comorbidities, disease duration or severity) in which eculizumab, inebilizumab, or satralizumab may be beneficial -
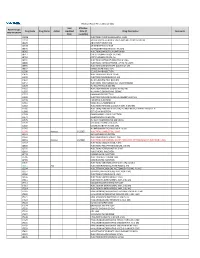
2021 Prior Authorization List Part B Appendix a (PDF)
Medicare Part B PA List Effective 2021 Last Effective Part B Drugs: Drug Code Drug Name Action Updated Date (if Drug Description Comments STEP THERAPY Date available) C9050 INJECTION, EMAPALUMAB-LZSG, 1 MG C9122 MOMETASONE FUROATE SINUS IMPLANT 10 MCG SINUVA J0129 ABATACEPT INJECTION J0178 AFLIBERCEPT INJECTION J0570 BUPRENORPHINE IMPLANT 74.2MG J0585 INJECTION,ONABOTULINUMTOXINA J0717 CERTOLIZUMAB PEGOL INJ 1MG J0718 CERTOLIZUMAB PEGOL INJ J0791 INJECTION CRIZANLIZUMAB-TMCA 5 MG J0800 INJECTION, CORTICOTROPIN, UP TO 40 UNITS J0896 INJECTION LUSPATERCEPT-AAMT 0.25 MG J0897 DENOSUMAB INJECTION J1300 ECULIZUMAB INJECTION J1428 INJECTION ETEPLIRSEN 10 MG J1429 INJECTION GOLODIRSEN 10 MG J1442 INJ FILGRASTIM EXCL BIOSIMIL J1447 INJECTION, TBO-FILGRASTIM, 1 MICROGRAM J1459 INJ IVIG PRIVIGEN 500 MG J1555 INJECTION IMMUNE GLOBULIN 100 MG J1556 INJ, IMM GLOB BIVIGAM, 500MG J1557 GAMMAPLEX INJECTION J1558 INJECTION IMMUNE GLOBULIN XEMBIFY 100 MG J1559 HIZENTRA INJECTION J1561 GAMUNEX-C/GAMMAKED J1562 INJECTION; IMMUNE GLOBULIN 10%, 5 GRAMS J1566 INJECTION, IMMUNE GLOBULIN, INTRAVENOUS, LYOPHILIZED (E.G. P J1568 OCTAGAM INJECTION J1569 GAMMAGARD LIQUID INJECTION J1572 FLEBOGAMMA INJECTION J1575 INJ IG/HYALURONIDASE 100 MG IG J1599 IVIG NON-LYOPHILIZED, NOS J1602 GOLIMUMAB FOR IV USE 1MG J1745 INJ INFLIXIMAB EXCL BIOSIMILR 10 MG J1930 Remove 1/1/2021 INJECTION, LANREOTIDE, 1 MG J2323 NATALIZUMAB INJECTION J2350 INJECTION OCRELIZUMAB 1 MG J2353 Remove 1/1/2021 INJECTION, OCTREOTIDE, DEPOT FORM FOR INTRAMUSCULAR INJECTION, 1 MG J2357 INJECTION, OMALIZUMAB, -

Middle Level IM-MS and CIU Experiments for Improved Therapeutic Immunoglobulin Subclass Fingerprinting ACS Paragon Plus Environment Analytical Chemistry T
Middle level IM-MS and CIU experiments for improved therapeutic immunoglobulin subclass fingerprinting ACS Paragon Plus Environment Analytical Chemistry T. Botzanowski, O. Hernandez-Alba, M. Malissard, E. Wagner-Rousset, E. Desligniere, O. Colas, F. Haeuw J., A. Beck, S. Cianferani To cite this version: T. Botzanowski, O. Hernandez-Alba, M. Malissard, E. Wagner-Rousset, E. Desligniere, et al.. Middle level IM-MS and CIU experiments for improved therapeutic immunoglobulin subclass fingerprinting ACS Paragon Plus Environment Analytical Chemistry. Analytical Chemistry, American Chemical Society, 2020, 92 (13), pp.8827-8835. 10.1021/acs.analchem.0c00293. hal-02960844 HAL Id: hal-02960844 https://hal.archives-ouvertes.fr/hal-02960844 Submitted on 8 Oct 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Analytical Chemistry This document is confidential and is proprietary to the American Chemical Society and its authors. Do not copy or disclose without written permission. If you have received this item in error, notify the sender and delete all copies. -

Submission of Eculizumab for NMOSD to PBAC Meeting November 2020
PBAC Secretariat MDP 952 Department of Health and Ageing GPO Box 9848 Canberra ACT 2601 7 October 2020 Re: Submission of eculizumab for NMOSD to PBAC meeting November 2020 This is a joint submission to the Pharmaceutical Benefits Advisory Committee (PBAC) in relation to eculizumab (Soliris) for neuromyelitis optica spectrum disorder (NMOSD) from MS Research Australia, the Centre for Community-Driven Research and MS Australia. • MS Research Australia is the largest national not-for-profit organisation dedicated to funding MS discoveries and coordinating MS research in Australia. • The Centre for Community-Driven Research is a non-profit organisation with expertise in gathering patient experience and expectations data. • MS Australia is the national voice for people with multiple sclerosis. MS Australia works in advocacy and communications and collaborates with their stakeholders to benefit thousands of people affected by MS across the country. MS Research Australia and MS Australia are writing to support the inclusion of eculizumab on the Pharmaceutical Benefits Scheme (PBS) for people with NMOSD. The Centre for Community-Driven Research is keen to inform the PBAC about the experience of people with NMOSD and their expectations of new treatments. The NMOSD community in Australia is not represented by a national peak body and as NMOSD and MS have some similarities, we are proud to advocate on behalf of those living with NMOSD. One area we are all particularly passionate about is the provision of affordable and accessible treatments that can improve the lives of people with NMOSD. About NMOSD NMOSD is a recently defined inflammatory disorder of the central nervous system (CNS) that was previously either misdiagnosed as MS or identified as Devic’s disease. -

Cimzia (Certolizumab Pegol) AHM
Cimzia (Certolizumab Pegol) AHM Clinical Indications • Cimzia (Certolizumab Pegol) is considered medically necessary for adult members 18 years of age or older with moderately-to-severely active disease when ALL of the following conditions are met o Moderately-to-severely active Crohn's disease as manifested by 1 or more of the following . Diarrhea . Abdominal pain . Bleeding . Weight loss . Perianal disease . Internal fistulae . Intestinal obstruction . Megacolon . Extra-intestinal manifestations: arthritis or spondylitis o Crohn's disease has remained active despite treatment with 1 or more of the following . Corticosteroids . 6-mercaptopurine/azathioprine • Certollizumab pegol (see note) is considered medically necessary for persons with active psoriatic arthritis who meet criteria in Psoriasis and Psoriatic Arthritis: Biological Therapies. • Cimzia, (Certolizumab Pegol), alone or in combination with methotrexate (MTX), is considered medically necessary for the treatment of adult members 18 years of age or older with moderately-to-severely active rheumatoid arthritis (RA). • Cimzia (Certolizumab pegol is considered medically necessary for reducing signs and symptoms of members with active ankylosing spondylitis who have an inadequate response to 2 or more NSAIDs. • Cimzia (Certolizumab Pegol) is considered investigational for all other indications (e.g.,ocular inflammation/uveitis; not an all-inclusive list) because its effectiveness for indications other than the ones listed above has not been established. Notes • There are several brands of targeted immune modulators on the market. There is a lack of reliable evidence that any one brand of targeted immune modulator is superior to other brands for medically necessary indications. Enbrel (etanercept), Humira (adalimumab), Remicade (infliximab), Simponi (golimumab), Simponi Aria (golimumab intravneous), and Stelara (ustekinumab) brands of targeted immune modulators ("least cost brands of targeted immune modulators") are less costly to the plan.