Cooperative Breeding Under Anthropogenic Habitat Change
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cooperative Breeding Shapes Post‐Fledging Survival in An
Received: 17 August 2016 | Revised: 2 December 2016 | Accepted: 22 December 2016 DOI: 10.1002/ece3.2744 ORIGINAL RESEARCH Cooperative breeding shapes post-fledging survival in an Afrotropical forest bird Dries Van de Loock1,2,3 | Diederik Strubbe1 | Liesbeth De Neve1 | Mwangi Githiru2,4 | Erik Matthysen3 | Luc Lens1 1Terrestrial Ecology Unit, Ghent University, Ghent, Belgium Abstract 2Department of Zoology, National Museums For avian group living to be evolutionary stable, multiple fitness benefits are expected. of Kenya, Nairobi, Kenya Yet, the difficulty of tracking fledglings, and thus estimating their survival rates, limits 3Evolutionary Ecology Group, University of our knowledge on how such benefits may manifest postfledging. We radio- tagged Antwerp, Campus Drie Eiken, Wilrijk, Belgium 4Wildlife Works, Voi, Kenya breeding females of the Afrotropical cooperatively breeding Placid greenbul (Phyllastrephus placidus) during nesting. Tracking these females after fledging permit- Correspondence Dries Van de Loock, Terrestrial Ecology Unit, ted us to locate juvenile birds, their parents, and any helpers present and to build in- Ghent University, K.L. Ledeganckstraat 35, dividual fledgling resighting datasets without incurring mortality costs or causing 9000 Ghent, Belgium Email: [email protected] premature fledging due to handling or transmitter effects. A Bayesian framework was used to infer age- specific mortality rates in relation to group size, fledging date, mater- Funding information Fonds Wetenschappelijk Onderzoek, Grant/ nal condition, and nestling condition. Postfledging survival was positively related to Award Number: G.0308.13N. group size, with fledglings raised in groups with four helpers showing nearly 30% higher survival until independence compared with pair- only offspring, independent of fledging date, maternal condition or nestling condition. -

Species List
May 11 – 23, 2019 Spain: Birding and Nature Tour With: Christine, Laura, Brad, Cathy, Elizabeth, and Richard (HO)= Distinctive enough to be counted as heard only (I)=introduced Tour Summary: What an amazing destination! A journey through Spain is an experience one will never forget. Over the course of this tour, we explored wildlife rich areas from the lofty peaks of the Gredos Mountains to the mudflats of Andalusia, from the cork oak forests of Extremadura to the saline pools of Castilla-La Mancha. We recorded 188 bird species in that time – species such as Great Bustard, Egyptian Vulture, Eurasian Hoopoe, European Roller, Crested Tit, Great Spotted Cuckoo, Pin-tailed Sandgrouse, and more. In the end, there was no agreement as to the favorite bird of the tour – each person selected three completely different birds! It was just not the birds that captivated our group. It was the richness of Spain’s culture, history, architecture, and cuisine interwoven with the natural landscape. BIRDS (188 species recorded, 3 heard only): DUCKS, GEESE AND SWANS: Anatidae (10) Graylag Goose Anser anser—the ancestor of the domestic goose and quite common in winter in Spain, but only a small percentage remain to breed – a couple lingering birds at Dehesa Abajo in Andalusia and a family of adults and goslings at Laguna Navaseca in Castille-La Mancha Common Shelduck Tadorna tadorna—very handsome shelduck, associated with saline wetlands; our best views were at the various lagoons in Castille-La Mancha, where they were quite common Northern Shovler Spatula -

La Mancha, Coto Donana & Extremadura 2017
Field Guides Tour Report Spain: La Mancha, Coto Donana & Extremadura 2017 May 6, 2017 to May 18, 2017 Chris Benesh & Godfried Schreur For our tour description, itinerary, past triplists, dates, fees, and more, please VISIT OUR TOUR PAGE. Spectacular skies greeted us during our visit to old Trujillo in the heart of Extremadura. Photo by guide Chris Benesh. So many birds around that you don´t know which to choose and observe. Do you recognize this feeling? We experienced many of these exciting moments in Spain during the Field Guides tour in May. It started straight away, on the first day, overlooking the natural lagoons of La Mancha Húmeda, where we had the chance to observe a great variety of species of ducks, grebes, terns, and passerines. The highlights here were the White-headed Duck, Eared Grebe, Red-crested Pochard, Whiskered Tern and Penduline Tit. In the National Park of Coto Donana again we found ourselves surrounded by birds: larks, bee-eaters, flamingos, Great Reed Warblers, Glossy Ibis, Squacco and Purple herons and a surprisingly well showing Little Bittern. With a bit of searching, scanning and listening we were able to also detect Red-knobbed Coot, Marbled Teal and Isabelline (Western Olivaceous) Warbler. Later in the week, close to Trujillo (Extremadura), we all enjoyed the excursion on the open, rolling plains, with Great and Little bustards, Eurasian Roller, Hoopoe, Calandra Lark, Montagu´s Harrier and many, many White Storks. For the shy Black Storks we had to wait one day more. In Monfrague National Park we discovered 3 pairs nesting on the breathtaking cliff of Peña Falcón. -
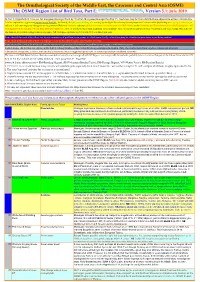
ORL 5.1 Hypothetical Spp Final Draft01a.Xlsx
The Ornithological Society of the Middle East, the Caucasus and Central Asia (OSME) The OSME Region List of Bird Taxa, Part E: , Version 5.1: July 2019 In Part E, Hypothetical Taxa, we list non-passerines (prefixed by 'N') first, then passerines (prefixed by 'P'). Such taxa may be from distributions adjacent to or have extended to A fuller explanation is given in Explanation of the ORL, but briefly, Bright green shading of a row (eg Syrian Ostrich) indicates former presence of a taxon in the OSME Region. Light gold shading in column A indicates sequence change from the previous ORL issue. Red font indicates added information since the previous ORL version or the Conservation Threat Status (Critically Endangered = CE, Endangered = E, Vulnerable = V and Data Deficient = DD only). Not all synonyms have been examined. Serial numbers (SN) are merely an administrative convenience and may change. Please do not cite them in any formal correspondence or papers. NB: Compass cardinals (eg N = north, SE = southeast) are used. Rows shaded thus and with yellow text denote summaries of problem taxon groups in which some closely-related taxa may be of indeterminate status or are being studied. Rows shaded thus and with yellow text indicate recent or data-driven major conservation concerns. Rows shaded thus and with white text contain additional explanatory information on problem taxon groups as and when necessary. English names shaded thus are species on BirdLife Tracking Database, http://seabirdtracking.org/mapper/index.php. Only a few individuals from very few colonies are involved. A broad dark orange line, as below, indicates the last taxon in a new or suggested species split, or where sspp are best considered separately. -

Southern Spain: the Europe Introtour April 2019
Tropical Birding Trip Report Southern Spain: The Europe Introtour April 2019 A Tropical Birding set departure tour SOUTHERN SPAIN: The Europe Introtour 1st – 9th April 2019 Tour Leader: Emma Juxon All photographs in this report were taken by Emma Juxon unless otherwise stated, species depicted in photographs are named in BOLD RED www.tropicalbirding.com +1-409-515-9110 [email protected] Tropical Birding Trip Report Southern Spain: The Europe Introtour April 2019 Introduction For European birders, there is nowhere better to take a birding trip than the Iberian Peninsula. A region largely overlooked by North American birders in the past, it boasts some of THE best birding on the continent, it’s certainly not to be missed. We visit the outstanding regions of Extremadura and Andalucía on this tour, making our way through breathtaking mountainous landscapes, through rolling steppes and spectacular marismas. With many participants visiting the Old World for the first time, it promises a wealth of lifers, great food, fantastic people and an easy-going introduction to the Mediterranean way of life. One of the many beauties of this tour is that we only have two bases. Starting in Madrid, we make our way through the beautiful Spanish countryside, passing vineyards and castillos to get to wildflower- carpeted Extremadura. Here we enjoy the Belen Steppe, Caceres Plains and the exceptional Monfragüe National Park, encountering incredible birds such as Eurasian Griffon, Pin-tailed Sandgrouse, Great Bustard and Iberian Magpie. From here we head south to our next base in the picturesque pilgrimage town of El Rocío; I love this charming place, with its sandy roads, wonderful bird-filled marshes and charismatic people. -

Tanzania, 30 November to 21 December 2020
Tanzania, 30 November to 21 December 2020 Thomas Pettersson This tour was organized by Tanzania Birding and Beyond Safaris and unfortunately, I was the only participant as my friend was prevented from going as was planned. The flights from Stockholm via Addis Ababa to Dar es Salaam and back with Ethiopian Airlines were uneventful. The only differences from my previous flights were that wearing face masks on the aircrafts was mandatory, and recommended at the airports, and that they checked my body temperature at both arrival and departure. I am not sure what the consequences would have been in case of fever. You must also complete a health declaration both for transfer and arrival. The outbound flight from Stockholm to Addis Ababa was about half empty and on the return perhaps only 25 % of the seats were occupied, which meant good nights sleep on three seats both ways. The flights between Addis Ababa and Dar es Salaam were fully booked both ways. The domestic flights with Coastal Aviation from Dar es Salaam via Zanzibar (Unguja) to Pemba and on to Tanga were also smooth, with the same regulations as above. The aircrafts were painfully small though, 12 seats. Not much space for legs and hand luggage. On the other hand, the distances are short. All in all, the tour was a big success. Accommodation was generally good, although basic at some places, but nothing to complain about. Food was excellent and plentiful, and I had no issues with the stomach. The drivers and the guides were excellent, in particular the outstanding Anthony, who guided most of the tour. -

Malawi Trip Report 2011
Malawi Trip Report 2011 Stanley Bustard (Denham's) in Nyika National Park 1(30) Malawi Trip Report, 30 October - 12 November 2011 A birdwatching tour perfectly arranged by Birding Africa (http://www.birdingafrica.com). Participants: Joakim, Elisabeth, Adrian (15) and Nova (10) Djerf from Östhammar, Sweden. Areas visited: Dzalanyama, Viphya Plateau, Nyika NP, Lake Malawi, Zomba and Liwonde NP. Total number of bird species recorded: 319 Total number of mammal species recorded: 23 Top 10 birds (our experience): 1. Pennant-winged Nightjar 2. Stanley Bustard (Denham's) 3. Cholo Alethe 4. Bar-tailed Trogon 5. White-winged Apalis 6. Anchieta's Sunbird 7. Livingstone's Flycatcher 8. Thick-billed Cuckoo 9. Boehm's Bee-eater 10. White-starred Robin Important literature and sound recordings: ✓ Southern African Birdfinder (Callan Cohen, Claire Spottiswoode, Jonathan Rossouw) ✓ The Birds of Malawi (Francoise Dowsett-Lemaire, Robert J. Dowsett) ✓ Birds of Africa south of the Sahara (Ian Sinclair, Peter Ryan) ✓ Bradt Guide: Malawi (Philip Briggs) ✓ The Kingdon pocket guide to African Mammals (Jonathan Kingdon) ✓ Sounds of Zambian Wildlife (Robert Stjernstedt) General about the trip: This was, with two exceptions (Zomba and Liwonde), a self catering trip. Self catering in Malawi means you bring your own food to the accommodation but get it prepared by the always present local cook and (often) his one or two assistants. This worked perfectly well in every place we stayed. All meals were thoroughly prepared and tasted very good. None of us had any stomach problems at all in the whole trip and we ate everything we was served. We do recommend this arrangement when travelling in Malawi. -
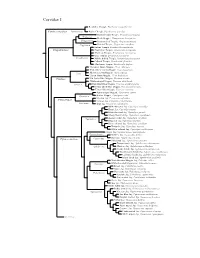
Corvidae Species Tree
Corvidae I Red-billed Chough, Pyrrhocorax pyrrhocorax Pyrrhocoracinae =Pyrrhocorax Alpine Chough, Pyrrhocorax graculus Ratchet-tailed Treepie, Temnurus temnurus Temnurus Black Magpie, Platysmurus leucopterus Platysmurus Racket-tailed Treepie, Crypsirina temia Crypsirina Hooded Treepie, Crypsirina cucullata Rufous Treepie, Dendrocitta vagabunda Crypsirininae ?Sumatran Treepie, Dendrocitta occipitalis ?Bornean Treepie, Dendrocitta cinerascens Gray Treepie, Dendrocitta formosae Dendrocitta ?White-bellied Treepie, Dendrocitta leucogastra Collared Treepie, Dendrocitta frontalis ?Andaman Treepie, Dendrocitta bayleii ?Common Green-Magpie, Cissa chinensis ?Indochinese Green-Magpie, Cissa hypoleuca Cissa ?Bornean Green-Magpie, Cissa jefferyi ?Javan Green-Magpie, Cissa thalassina Cissinae ?Sri Lanka Blue-Magpie, Urocissa ornata ?White-winged Magpie, Urocissa whiteheadi Urocissa Red-billed Blue-Magpie, Urocissa erythroryncha Yellow-billed Blue-Magpie, Urocissa flavirostris Taiwan Blue-Magpie, Urocissa caerulea Azure-winged Magpie, Cyanopica cyanus Cyanopica Iberian Magpie, Cyanopica cooki Siberian Jay, Perisoreus infaustus Perisoreinae Sichuan Jay, Perisoreus internigrans Perisoreus Gray Jay, Perisoreus canadensis White-throated Jay, Cyanolyca mirabilis Dwarf Jay, Cyanolyca nanus Black-throated Jay, Cyanolyca pumilo Silvery-throated Jay, Cyanolyca argentigula Cyanolyca Azure-hooded Jay, Cyanolyca cucullata Beautiful Jay, Cyanolyca pulchra Black-collared Jay, Cyanolyca armillata Turquoise Jay, Cyanolyca turcosa White-collared Jay, Cyanolyca viridicyanus -

Ultimate Kenya
A pair of fantastic Sokoke Scops Owls. (DLV). All photos taken by DLV during the tour. ULTIMATE KENYA 1 – 20 / 25 APRIL 2017 LEADER: DANI LOPEZ-VELASCO Kenya lived up to its reputation of being one of the most diverse birding destinations on our planet. Once again, our Ultimate Kenya recorded a mind-boggling total of more than 750 species. This was despite the fact that we were prioritizing Kenyan specialities (a task in which we were extremely successful) rather than going all out for a huge list! 1 BirdQuest Tour Report: Ultimate Kenya www.birdquest-tours.com The first leg of our epic adventure saw us focusing on the Arabuko-Sokoke Forest where the birding is tough but the rewards are great. Over the course of the two and a half days our talented local guide helped us find all of the main specialities, with the exception of the difficult Clarke’s Weavers, which were presumably on their recently discovered breeding grounds in marshes to the north. Crested Guineafowl and Northern Carmine Bee-eater. We spent much time creeping along sandy tracks, gradually finding our targets one by one. We succeeded in getting great views of a number of skulkers, including a rather showy East Coast Akalat on our last afternoon, some reclusive Eastern Bearded Scrub Robins, a very obliging Red-tailed Ant Thrush and skulking Fischer’s and Tiny Greenbuls. Once in the Brachystegia we kept our eyes and ears open for roving flocks of flock-leader Retz’s and Chestnut-fronted Helmet Shrikes, and with these we found awkward Mombasa Woodpeckers and a single Green-backed Woodpecker, and a variety of smaller species including Black-headed Apalis, Green Barbet, Eastern Green Tinkerbird, dainty Little Yellow Flycatchers, Forest Batis, Pale Batis, cracking little Amani and Plain-backed Sunbirds and Dark-backed Weaver. -

Seville and the Alentejo David Bradnum, Shaun Harvey & Howard Vaughan – June 2019
Seville and the Alentejo David Bradnum, Shaun Harvey & Howard Vaughan – June 2019 Overview and Logistics This was a short, low-cost birding break to Spain and Portugal. We had two aims: to see a handful of late-arriving spring migrants – White-rumped Swift, Western Olivaceous Warbler and Rufous-tailed Scrub-robin – and to enjoy as many as possible of the local specialities on the Castro Verde plains and the Rio Guadiana valley. We flew Easyjet from Gatwick to Seville. This cost c. £140 return each, booked just over a month in advance of the trip. We hired a car from Europcar, via Easyjet. This turned out to be a Skoda Octavia diesel for a bargain £65. Collection and drop-off were both relatively quick and fuss-free. We stayed in an Airbnb rental in the tiny Portuguese village of Bens, east of Mertola, for three nights. This was very convenient for the White-rumped Swift site and also another bargain, coming in at a total of just £130 for three nights! The traditional cottage was very comfortable and peaceful, with Iberian Magpies outside during the day and Red-necked Nightjar calling (once, at least!) during the night. We self-catered throughout, contributing to an overall cost of around £270 per person for the entire trip. The weather was generally good, with plenty of sunshine and peak temperature around 32°C (probably a little lower than might be expected). Day three was unusually overcast and even a little rainy around lunchtime – though this was actually helpful in that we could keep birding through the warmest part of the day! Ahead of the trip, we used the Finding Birds in Southern Portugal Gosney site guide to identify the best sites, and then topped this up with more recent info from eBird. -

Spain - Realm of the Iberian Lynx
Spain - Realm of the Iberian Lynx Naturetrek Tour Report 19 - 24 January 2020 Iberian Lynx Iberian Lynx Cinereous Vulture Sunset Report compiled by Niki Williamson Images by Simon Tonkin Naturetrek Mingledown Barn Wolf's Lane Chawton Alton Hampshire GU34 3HJ UK T: +44 (0)1962 733051 E: [email protected] W: www.naturetrek.co.uk Tour Report Spain - Realm of the Iberian Lynx Tour participants: Simon Tonkin & Niki Williamson (leaders) with 13 Naturetrek clients.. Summary For our band of patient nature-lovers, this six-day exploration of the realm of the Iberian Lynx gave us something amazing every day! Six different individuals of the endangered Spanish Imperial Eagle, rare Marbled Ducks, Hawfinches, Spanish Ibex cantering across a rock face, herds of Red Deer swimming a lake, duetting Little Owls, clouds of Cinereous and Griffon Vultures, Golden Eagles and shades of blue in the form of Bluethroat, Blue Rock Thrush, Iberian Magpie and Common Kingfisher were just some of our trip´s natural highlights. Our hosts´ hospitality was fantastic at both bases, and the group enjoyed sampling delicious local food such as chickpea and spinach stew, salmorejo soup and egg revuelto dishes, not to mention mouth-watering picnics in the sun, sometimes accompanied by dazzling flocks of Iberian Magpies, always accompanied by wine! Our fleeting glimpse of a female Iberian Lynx in Doñana Natural Park was to provide a suitable appetite-whetter for our superb encounter in Sierra de Morena, where a stunning female stalked across the track in front of us before taking up a pose on a nearby rock, allowing us to watch for over an hour! Day 1 Sunday 19th January Leaders Simon and Niki met the group as they converged on Sevilla airport, from various flights and pre-trip stays. -

Zambia and Malawi Trip Report – August/September 2014
Zambia and Malawi Trip Report – August/September 2014 Miombo Tit www.birdingecotours.com [email protected] 2 | T R I P R E P O R T Zambia and Malawi August/September 2014 This trip was run as a customized tour for three clients, all with lists of well over 7000 species seen worldwide, and in fact Dollyann was hoping to reach 8000 species by the end of this trip. Travel to some really remote destinations, particularly in Malawi, was necessary to find some of the group’s target birds. Places like Misuku Hills and Uzumara Forest in Malawi are hardly ever visited by birders, primarily from a logistics point of view, and also because of lack of suitable accommodation. Both these destinations are, however, excellent birding spots, and Uzumara in particular could be included in most itineraries, using accommodation in the town of Rumphi as a base. On the Zambian side we included the Mwinilunga area, a must for any serious birder; this area hosts many Angolan/Congo specials, found nowhere else in Zambia. Day 1, 14th August. Livingstone Airport to Lodge Ron, Dollyann, and Kay arrived on the same flight from Johannesburg at around 13h00. After a short meet and greet and a quick visit to the bank for some local currency, we loaded up and started our journey to our lodge. Not much was seen en route other than a few marauding Pied Crows and a single African Grey Hornbill. We arrived at the lodge in good time and decided to take 20 minutes to refresh, before starting our bird quest.