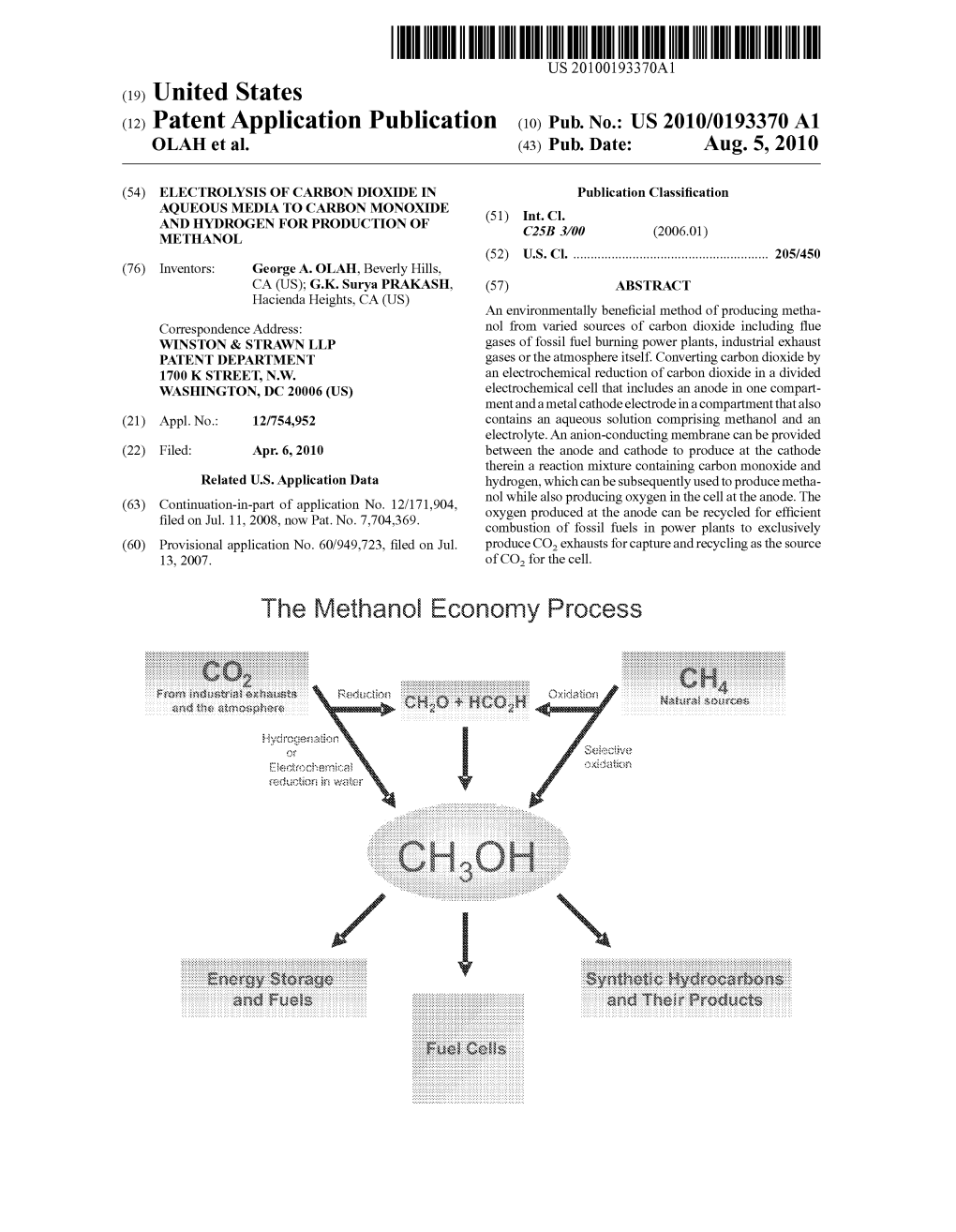
The Methanol Economy Process
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

HYDROGEN ECONOMY VS. METHANOL ECONOMY SARTHAK TIBDEWAL, UTSAV SAXENA and ANAND V
Int. J. Chem. Sci.: 12(4), 2014, 1478-1486 ISSN 0972-768X www.sadgurupublications.com HYDROGEN ECONOMY VS. METHANOL ECONOMY SARTHAK TIBDEWAL, UTSAV SAXENA and ANAND V. P. GURUMOORTHY* Chemical Engineering Division, School of Mechanical and Building Sciences, VIT University, VELLORE – 632014 (T.N.) INDIA ABSTRACT Today, the human civilization is very much dependent on fossil fuels, which make the blood and bone of this modern world. These precious natural resources, which form over the course of hundreds of years, are being consumed swiftly. In this alarming situation, it is very much necessary to think for a replacement, which fulfils the social needs without disturbing the environmental stability. One such approach discussed most over the years is ‘Hydrogen economy-Producing and using hydrogen as a clean fuel’, but there is no infrastructure for it. As it is a volatile gas, so it needs to be handled and stored at high a pressure. Moreover, it is an inflammable gas, which makes its usage as a transportation fuel difficult. A more potential and reasonable alternative, which is gaining importance is ‘Methanol Economy’ where methanol can be used as a source for transportation, energy storage and raw material for artificial hydrocarbons and their commodities. It is an excellent fuel. Methanol prices today are competitive with hydrocarbon fuels (on energy basis). Development is noted on the commercial conversion of biomass to methanol by means of thermochemical mechanism. Adequate feedstock of natural gas and coal lies to empower the handling of exhaustible methanol as transition fuel to renewable methanol from biomass. This paper discusses methanol’s potential as an alternate of the hydrogen economy. -

Renewable Methanol Report Renewable Methanol Report
In association with: Renewable Methanol Report Renewable Methanol Report Renewable Methanol Report This report has been produced by ATA Markets Intelligence S.L. on behalf of the Methanol Institute. The information and opinions in this report were prepared by ATA Markets Intelligence and its partners. ATA Markets Intelligence has no obligation to tell you when opinions or information in this report change. Publication Date: December 2018 ATA Markets Intelligence makes every effort to use reliable, comprehensive information, but we make no representation that it is accurate or complete. In no event shall ATA Markets Intelligence and its partners be liable for any damages, Authors: losses, expenses, loss of data, loss of opportunity or profit caused by the use of the material or contents of this report. Charlie Hobson ATA Insights is a brand of ATA Markets Intelligence, whose registered office is located in Calle Serrano, 8, 3º izquierda, Carlos Márquez (editor) Post-code 28001, Madrid, Spain. Registered in the Mercantile Registry of Madrid, CIF number B87725198. Design: © Methanol Institute 2018 Henrik Williams www.methanol.org 2 Renewable Methanol Report CONTENTS Executive Summary................................................................................. 5 Why consider renewable methanol? .................................................7 Legislation drives change.....................................................................................7 Report structure ..................................................................................................8 -

Technoeconomics and Sustainability of Renewable Methanol and Ammonia Productions Using Wind Power-Based Hydrogen Michael J
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Chemical and Biomolecular Research Papers -- Yasar Demirel Publications Faculty Authors Series 2015 Technoeconomics and Sustainability of Renewable Methanol and Ammonia Productions Using Wind Power-based Hydrogen Michael J. Matzen University of Nebraska-Lincoln, [email protected] Mahdi H. Alhajji University of Nebraska-Lincoln, [email protected] Yasar Demirel University of Nebraska - Lincoln, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/cbmedemirel Part of the Biochemical and Biomolecular Engineering Commons, Biomedical Engineering and Bioengineering Commons, and the Thermodynamics Commons Matzen, Michael J.; Alhajji, Mahdi H.; and Demirel, Yasar, "Technoeconomics and Sustainability of Renewable Methanol and Ammonia Productions Using Wind Power-based Hydrogen" (2015). Yasar Demirel Publications. 9. http://digitalcommons.unl.edu/cbmedemirel/9 This Article is brought to you for free and open access by the Chemical and Biomolecular Research Papers -- Faculty Authors Series at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Yasar Demirel Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. hemical E C n d g e in c e Matzen et al., J Adv Chem Eng 2015, 5:3 n e a r i v n d g A Advanced Chemical Engineering http://dx.doi.org/10.4172/2090-4568.1000128 ISSN: 2090-4568 Research Article Open Access Technoeconomics and Sustainability of Renewable Methanol and Ammonia Productions Using Wind Power-based Hydrogen Michael Matzen, Mahdi Alhajji and Yaşar Demirel* Department of Chemical and Biomolecular Engineering, University of Nebraska Lincoln, Lincoln NE 68588, USA Abstract This study analyzes and compares the economics and sustainability aspects of two hydrogenation processes for producing renewable methanol and ammonia by using wind-power based electrolytic hydrogen. -

Moving Ahead from Hydrogen to Methanol Economy: Scope and Challenges
Moving ahead from Hydrogen to Methanol Economy: Scope and challenges Ankit Sonthalia SRMIST: SRM Institute of Science and Technology Naveen Kumar Delhi Technological University Mukul Tomar Delhi Technological University Edwin Geo V ( [email protected] ) SRM University https://orcid.org/0000-0002-7303-3984 Thiyagarajan S SRM Institute of Science and Technology Arivalagan Pugazhendhi Ton Duc Thang University Research Article Keywords: Hydrogen, methanol, biomass, carbon dioxide, syngas, gasication Posted Date: March 4th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-280230/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Moving ahead from Hydrogen to Methanol Economy: Scope and challenges 1,2 2 2 3* Ankit Sonthalia , Naveen Kumar , Mukul Tomar , Varuvel Edwin Geo , Subramanian Thiyagarajan3 and Arivalagan Pugazhendhi4 1Department of Automobile Engineering, SRM Institute of Science and Technology, NCR Campus, Ghaziabad – 201204, India 2Center for Advanced Studies and Research in Automotive Engineering, Delhi Technological University, Bawana Road, Delhi – 110042, India 3Green Vehicle Technology Research Centre, Department of Automobile Engineering, SRM Institute of Science and Technology, Kattankulathur, Tamil Nadu – 603203, India 4Innovative Green Product Synthesis and Renewable Environment Development Research Group, Faculty of Environment and Labour Safety, Ton Duc Thang University, Ho Chi Minh City, Vietnam. *Corresponding Author: [email protected] Abstract Energy is the driver in the economic development of any country. It is expected that the developing countries like India will account for 25% hike in world-wide energy demand by 2040 due to the increase in the per capita income and rapid industrialization. Most of the developing countries do not have sufficient oil reserves and imports nearly all of their crude oil requirement. -

Methanol As a Marine Fuel
METHANOL AS A MARINE FUEL Chris Chatterton, COO LNGgc ASIA 27 – 28 FEBRUARY 2019 NOVATEL, CLARKE QUAY, SINGAPORE WWW.METHANOL.ORG Contents - About MI - Developing Trends for New Capacity - Global Methanol Fuel Blending - Methanol as Marine Fuel WWW.METHANOL.ORG MEMBERS Tier 1 Tier 2 Tier 3 Ecofuel Tier 4 WWW.METHANOL.ORG DEVELOPING TRENDS 02 FOR NEW CAPACITY WWW.METHANOL.ORG WHERESUPPLY IS METHANOL CAPACITY PRODUCED? BY REGION • Merchant projects are focused in USGC • China future capacity additions may slow, with a focus on integrated facilities SupplySupply Capacity Capacity for for Methanol Methanol by Region 20122012 –- 2022E2022E 100,000 90,000 80,000 70,000 60,000 tonnes 50,000 - 000 - 40,000 30,000 20,000 10,000 - 2012 2013 2014 2015 2016 2017E 2018E 2019E 2020E 2021E 2022E 2012 2013 2014 2015 2016 2017 2018E 2019E 2020E 2021E 2022E Asia North America South America Europe Rest of World Middle East Source: MMSA WWW.METHANOL.ORG WHERECHINA IS METHANOLMETHANOL PRODUCED?DEMAND DRIVES WORLD MARKETS Projected Methanol Demand Growth, by Product & Region 2017– 2027E Source: MMSA WWW.METHANOL.ORG WHERERENEWABLE IS METHANOL PATHWAYS PRODUCED? MSF ВЕДЕТ СВОЮ ДЕЯТЕЛЬНОСТЬ В БОЛЕЕ ЧЕМ 60 СТРАНАХ МИРА WWW.METHANOL.ORG WHEREAN EFFICIENT IS METHANOL ENERGY PRODUCED? CARRIER WWW.METHANOL.ORG WHEREVARIOUS IS METHANOL STAGES OFPRODUCED? DEVELOPMENT Methanol category Commercial Feasibility and R&D Stopped or On-hold Bio-methanol • BioMCN (NL) • Biogo (GER) • BioMCN (glycerine) (NL) • Enerkem (CAN) • Enerkem (NL) • Chemrec (SE) • New Fuel (DEN) • LowLands -

Bio-Methanol: the Future Alternative for Both Fossil Fuels and Electric Drives?
Bio-Methanol: The future Alternative for both Fossil Fuels and Electric Drives? Report written by: Antonia Meckel Viborg, June 2019 Executive summary Methanol as motor fuel has gained interest in recent years due to its low price, easy handling and high octane number. Methanol can nowadays be produced from biogas which yields an extremely low Greenhouse Gas emission – easily comparable to those of electric vehicles. Biogas is also very easy to come by, especially in Denmark with its enormous industry in cattle and hog farms. Efforts to establish a large methanol factory in Denmark with connection to the Danish gas grid are ongoing. Until that happens methanol can easily be imported from Norway. Certificate trading ensures that the methanol is based on Danish biogas. Methanol is not yet implemented in the Danish transport sector. The current exchange agreement between the fuel companies only allows ethanol to be added to gasoline. If this barrier could be removed, temporary fuels such as A7 could be introduced. In the long term, however, completely new fuels are required. The Danish Methanol Association and the Danish Technological Institute participate in a project that tested a 105 Octane M85 fuel consisting of 85% methanol and 15% petrol. The pilot car, a Peugeot 107 “City Car”, got a Flex Fuel Kit installed and its engine performance went up by 5-7% with all emissions kept in place. M85’s emission of CO2 is an important point in this report, and especially the comparison with the CO2 “neutrality” of electric vehicles deserves the spotlight we have given it. -

The Methanol Alternative Robert Zubrin
REVIEWS & RECONSIDERATIONS The Methanol Alternative Robert Zubrin ny serious energy policy to all three dimensions of the energy must deal with three criti- problem. Acal issues. First, economic: There is no shortage of energy The policy must provide an ener- experts with grand designs and pro- gy resource base sufficient to allow posals—from technophile dreams of for continued worldwide economic an unworkable “hydrogen economy,” growth for the foreseeable future. to Malthusian calls for enforced eco- Second, environmental: The policy nomic limits through conservation, must be compatible with the long- to socialist schemes for creating term flourishing of life on Earth, massive government-subsidized syn- including human life and civilization. thetic-fuel industries, to the libertar- And finally, stra- ian faith in the tegic: The policy Beyond Oil and Gas: The Methanol Economy Invisible Hand. must ensure that By George A. Olah, Alain Goeppert, and Compared to G. K. Surya Prakash control of the Wiley-VCH ~ 2006 ~ 290 pp. such misguided Earth’s energy $29.95 (hardcover) alternatives, the resources, and competence and thus its future, lies in the hands of free rationality of The Methanol Economy societies committed to human prog- is refreshing. ress, and taken away from tyrannical The authors begin by describing the and terrorism-promoting states. dimensions of the worldwide energy George Olah, recipient of the 1994 problem: Even as our reserves of fos- Nobel Prize in Chemistry, is one of sil fuels have grown in recent decades, the giants of twentieth-century sci- the demand is growing faster, and as ence, and his coauthors are solid tech- more of the world modernizes, a nical men. -

Overview of Global Methanol Fuel Blending
Overview of Global Methanol Fuel Blending Gregory Dolan, CEO – Methanol Institute Trinidad and Tobago Methanol Fuel Blending Forum 24 January 2019 WWW.METHANOL.ORG Methanol: Broad Feedstocks and Markets feedstock conversion derivatives products markets other solvents appliances chloromethanes ~65% MTO automotive natural gas methylamines DME construction biodiesel ~35% gasoline blending MTMA electronics MTBE coal acetic acid fuel methanol synthesis paint formaldehyde <1% pharma biomass & and renewables more…. WWW.METHANOL.ORG Methanol is a versatile fuel source Out of the ~80 million metric tons of methanol sold globally in 2018, energy and fuel uses represent 40% of total demand FUELS TECHNOLOGIES • Neat fuel • SI & CI engines • Low blends • Turbines • High blends • Fuel cells • GEM • MTBE SEGMENTS • Biodiesel • Road & non-road transportation • DME & OME • Power & heat generation • MTG • Marine WWW.METHANOL.ORG Global Methanol Fuel Examples Sweden – marine Canada – Waterfront Iceland – M100 fuel vessels trials Denmark –fuel USA –motorsport cells for China– M15 to fuel UK – EN228 vehicles M100, boilers, low blend cook stoves Italy – Eni/FCA Israel – M15, power M15/E5 generation New Zealand – Egypt – M15 M3 trials Africa – cook stoves India – Methanol Economy roadmap Australia – GEM fuels WWW.METHANOL.ORG 01 ROAD TRANSPORT WWW.METHANOL.ORG Solutions for gasoline and diesel engines gasoline diesel Low blends FAME Mid level blends SI/CI HDVs High blends DME/OME WWW.METHANOL.ORG Various Gasoline/Diesel Blend Options M3 – M15 A20 – A30 M51-100 • EU allows M3 • Automakers call for • ASTM D5797 (EN228) higher octane to standard revision Blended a.o. in UK facilitate greater • M100 dedicated and NL engine efficiency vehicles (e.g. -

Alternative Fuels for Light-Duty Vehicles
Replacing Gasoline: Alternative Fuels for Light-Duty Vehicles September 1990 OTA-E-364 NTIS order #PB91-104901 Recommended Citation: U.S. Congress, Office of Technology Assessment, Replacing Gasoline: Alternative Fuels for Light-Duty Vehicles, OTA-E-364 (Washington, DC: U.S. Government Printing Office, September 1990). For sale by the Superintendent of Documents U.S. Government Printing Office, Washington, DC 20402-9325 (order form can be found in the back of this report) Foreword Among the several major issues that Congress has addressed in the process of reauthorizing the Clean Air Act, the future role of alternative highway transportation fuels in reducing urban smog is one of the more prone to argument. Past attempts to reduce pollution levels from highway vehicles have focused primarily on the vehicles themselves; adjustments to fuels were considered mainly when these were necessary to allow vehicular controls to work (eliminating lead from gasoline was necessary to avoid poisoning the catalytic converters on the vehicles). As vehicular emissions control efficiencies rose past 90 percent and further improvements became more difficult, however, attention turned to the idea that some alternatives to gasoline have combustion and/or other physical and chemical properties that might allow the achievement of ultra-low emissions levels. The fuels of interest include methanol (wood alcohol), ethanol (grain alcohol), natural gas, electricity, and hydrogen. In this report, requested by the House Committee on Energy and Commerce and the Senate Committee on Energy and Natural Resources, which is part of OTA’s ongoing assessment of Technological Risks and Opportunities in Future U.S. Energy Supply and Demand, OTA gives a broad overview of the qualities of the competing fuels and examines in depth some of the most contentious issues associated with the wisdom of active Federal support for introducing the fuels. -

The Methanol Economy
The Methanol Economy G. K. Surya Prakash Loker Hydrocarbon Research Institute and Department of Chemistry University of Southern California Los Angeles, CA 90089-1661 USA Sustainable Methanol: An Alternative Green Fuel for the Future Workshop IASS Potsdam, Germany November 22-23, 2011 World population (in millions) Projection 1650 1750 1800 1850 1900 1920 1952 2000 2050 * 545 728 906 1171 1608 1813 2409 6200 8000 to 11000 * Medium estimate. Source: United Nations, Population Division Petawatt-hours (10 15 watt-hours) 200 History Projections 189 180 175 162 v150 petawatt-hours ~ 15 terawatts 160 148 (15,000 power plants of 1 gigawatt) 140 121 120 107 102 v21 TW by 2025 100 91 84 80 71 61 v30 TW by 2050 60 40 20 0 1970 1975 1980 1985 1990 1995 2002 2010 2015 2020 2025 World Primary Energy Consumption, 1970 to 2025 Based on data from Energy information Administration (EIA) Coal More than 80% of our 26.0% energy comes from Other fossil fuels 0.6% Combustible Renewables & Waste Oil 10.1% 34.4% Hydro 2.2% Nuclear 6.2% Natural gas 20.5% Total 11 741 Mtoe Distribution of the World Total Primary Energy Supply in 2006. Based on data from the International Energy Agency (IEA) Key World Energy Statistics 2008 Increasing world population Increase in standard of living Increase in fossil fuel use Increase in carbon dioxide -Oil, gas, coal (hydrocarbons) content of the atmosphere Finite sources – non-renewable Greenhouse effect On the human timescale (Global warming). 390 ppm Hydrocarbon Sources 17th-19th Century - industrial revolution coal 19th Century -

The Potential Role of Hydrogen in India a Pathway for Scaling-Up Low Carbon Hydrogen Across the Economy
The Potential Role of Hydrogen in India A pathway for scaling-up low carbon hydrogen across the economy WILL HALL, THOMAS SPENCER, G RENJITH, SHRUTI DAYAL THE ENERGY AND THE ENERGYR ANESOURCED S INSTITUTE RCreatingESOURCE InnovativeS INSTITUTE Solutions for a Sustainable Future Creating Innovative Solutions for a Sustainable Future ©2020 The Energy and Resources Institute Authors Will Hall, Fellow, TERI Thomas Spencer, Fellow, TERI G Renjith, Research Associate, TERI Shruti Dayal, Project Associate, TERI Reviewer Mr Girish Sethi, Senior Director, TERI Advisor Dr Ajay Mathur, Director General, TERI Disclaimer This report is an output of a research exercise undertaken by TERI and supported by CIFF. It does not represent the views of the supporting organizations or the acknowledged individuals. While every effort has been made to avoid any mistakes or omissions, TERI would not be in any way liable to any persons/organizations by reason for any mistake or omission in the publication. Suggested Citation: Hall, W., Spencer, T., Renjith, G., and Dayal, S. 2020. The Potential Role of Hydrogen in India: A pathway for scaling-up low carbon hydrogen across the economy. New Delhi: The Energy and Resources Institute (TERI). Published by The Energy and Resources Institute (TERI) Darbari Seth Block, IHC Complex, Lodhi Road, New Delhi - 110 003 THE POTENTIAL ROLE OF HYDROGEN IN INDIA A PATHWAY FOR SCALING-UP LOW CARBON HYDROGEN ACROSS THE ECONOMY WILL HALL, THOMAS SPENCER, G RENJITH, SHRUTI DAYAL THE ENERGY AND RESOURCES INSTITUTE Creating Innovative Solutions for a Sustainable Future | 2 | THE POTENTIAL ROLE OF HYDROGEN IN INDIA ENERGY TRANSITIONS COMMISSION INDIA Energy Transitions Commission (ETC) India is a research platform based in The Energy and Resources Institute (TERI) in Delhi. -

Methanol: a Future Transport Fuel Based on Hydrogen and Carbon Dioxide?
Methanol: a future transport Science and Technology Options Assessment fuel based on hydrogen and carbon dioxide? Science and Technology Options Assessment European Parliamentary Research Service European Parliament PE 527.377 - April 2014 Methanol: a future transport fuel based on hydrogen and carbon dioxide? Economic viability and policy options Study IP/A/STOA/FWC/2008-096/Lot1/C1/SC3 April 2014 PE 527.377 STOA - Science and Technology Options Assessment The STOA project 'Methanol: a future transport fuel based on hydrogen and carbon dioxide' was carried out by ISIS (Institute of Studies for the Integration of Systems - Italy) project coordinator; together with Tecnalia (Spain). AUTHORS Stefano Faberi, Loriana Paolucci, reviewed by Andrea Ricci (ISIS) Daniela Velte, Izaskun Jiménez (Tecnalia) RESPONSIBLE ADMINISTRATOR Peter Ide-Kostic Science and Technology Options Assessment (STOA) Directorate for Impact Assessment and European Added Value Directorate-General for Parliamentary Research Services European Parliament, Rue Wiertz 60, B-1047 Brussels E-mail: [email protected] LINGUISTIC VERSION Original: EN ABOUT THE PUBLISHER To contact STOA or to subscribe to its newsletter please write to: [email protected] This document is available on the Internet at: http://www.ep.europa.eu/stoa/ DISCLAIMER The opinions expressed in this document are the sole responsibility of the authors and do not necessarily represent the official position of the European Parliament. Reproduction and translation for non-commercial purposes are authorised, provided the source is acknowledged and the publisher is given prior notice and sent a copy. Manuscript completed in February 2014 Brussels, © European Union, 2014 PE 527.377 ISBN 978-92-823-5529-9 DOI 10.2861/57305 CAT QA-01-14-284-EN-C Methanol: a future transport fuel based on hydrogen and carbon dioxide? Abstract This study discusses the technological, environmental and economic barriers for producing methanol from carbon dioxide, as well as the possible uses of methanol in car transport in Europe.