Full Text Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

KERALA) S.SYNUDEEN SAHIB Department of Zoology, S.N.College, Kollam-691001, Kerala, India
© 2017 JETIR February 2017, Volume 4, Issue 2 www.jetir.org (ISSN-2349-5162) FISH DIVERSITY IN THE KANJIRACODE KADAVU LAKE, KOLLAM (KERALA) S.SYNUDEEN SAHIB Department of Zoology, S.N.College, Kollam-691001, Kerala, India. ABSTRACT The fish samples for the present study were collected from the Kanjiracode Kadavu Lake, Kollam during September 2017 to February 2018. A total of 19 species belonging to 18 families and 19 genera were collected from the study area during the present study. Diversity of fishes and threats to the fauna are discussed Key words: Thevally kadavu, fish diversity, threats. INTRODUCTION Fishes, one of the important component in the aquatic ecosystem and plays a significant role in the economy. The tropical aquatic system bestowed with diverse fish species . They occupy a significant position in the socio – economic fabric of the south Asian countries by providing the population not only the nutrients food but also income and employment opportunities. Forty per cent of the total catch of fishes in India is obtained from the Kerala State. An expert committee constituted by the Govt. of Kerala has strongly advocated transformations of the backwaters, reservoirs, lakes, ponds ,tanks etc in to fish farms. Information on the abundance and distribution of fishes forms the basic step in assessing the fishery resources and their management in aquatic biotopes. A comprehensive survey of the fishery resources of the lakes, rivers etc of Kerala has become essential for the effective management, planned exploitation and scientific conservation of these highly productive water bodies. India is endowed with a vast expanse of open inland waters in the form of rivers, canals, estuaries, natural and man made lakes, back waters, mangrove wetlands. -

Particulars of Some Temples of Kerala Contents Particulars of Some
Particulars of some temples of Kerala Contents Particulars of some temples of Kerala .............................................. 1 Introduction ............................................................................................... 9 Temples of Kerala ................................................................................. 10 Temples of Kerala- an over view .................................................... 16 1. Achan Koil Dharma Sastha ...................................................... 23 2. Alathiyur Perumthiri(Hanuman) koil ................................. 24 3. Randu Moorthi temple of Alathur......................................... 27 4. Ambalappuzha Krishnan temple ........................................... 28 5. Amedha Saptha Mathruka Temple ....................................... 31 6. Ananteswar temple of Manjeswar ........................................ 35 7. Anchumana temple , Padivattam, Edapalli....................... 36 8. Aranmula Parthasarathy Temple ......................................... 38 9. Arathil Bhagawathi temple ..................................................... 41 10. Arpuda Narayana temple, Thirukodithaanam ................. 45 11. Aryankavu Dharma Sastha ...................................................... 47 12. Athingal Bhairavi temple ......................................................... 48 13. Attukkal BHagawathy Kshethram, Trivandrum ............. 50 14. Ayilur Akhileswaran (Shiva) and Sri Krishna temples ........................................................................................................... -

CRZ-3- Reported Violations(1)
Sl No 1 JosephS,Pandarathu 7 Josesunil.Aleena 6 Baiji,AlphaNivas, 5 JosephS, 4 Nelson,Kattil 3 Margrett, 2 Sindhu,Pallithekkathil, Eravipuram Eravipuram.P.O. Mayyanadu po,Kollam Purayidam, Tanni, Thekkumbhagam, mandiram, Thekkuumbhagam Adichamanthoppu, Tanni, Kollam Adhichamanthoppu, Purayidam, Mayyanadu Thekkumbhagam, Varupurayidam, Aravila ,Kavanad.P.O Name andadress of theowner Eravipuram Eravipuram Eravipuram Eravipuram Eravipuram Eravipuram Eravipuram village Ration card No. Res. Res. Res. Res. Res. Res. Res. Type of construction DETAILS OFUNAUTHERISEDCONTRUCTIONSINCRZAREA 1 1 1 1 1 1 1 New/re construction /Rennovation KOLLAM MUNICIPALCORPORATION 134/52, 1.19 747/14/2/2, 755/9, 138/2/9, 738/12/2/9/2 747/14/2-2, 748/1/66, 1.62are Survey no. and extent are 4.05are 3.98are 1.62 are , 1.52 are 4.05 are CRZ -IIPROFORMA-6/12/2019 Sea shore,Back sea/back water/ Sea shore Sea shore Sea shore Sea shore Sea shore Sea water thuruth/kayal II 110 m 34.02m2II 3.55 1 II 73.2m 53.56m II 53 II 175m 73.12m2II 3.55 69.55m 1 60.03m2II 3.55 131.2m 1 65.08m2 3.55 1 CRZ category 168.45 m m,108.7 5 m Distance from HTL 73.12m2 3.55 1 36.48 3.55 1 Area of 2 building Height of 3.55 1 Building Number of floors Present condition of Completed Completed Completed Completed Completed Completed Completed construction Reported Reported to Reported Reported Reported Reported Reported Action taken by the to KCZMA KCZMA to KCZMA to KCZMA to KCZMA to KCZMA to KCZMA local body Details Fisherman Fisherman Fisherman Fisherman Fisherman Fisherman Fisherman Fishermen/ Fishermen (G) Remarks Sl No 14 Joseph&Mable, 13 Vittty,Newcolony 12 Lalan&Vijayakumari, 11 Agnus, 10 Anu,Anu 15 Robin& Stepimole 8 Jayan&Anitha, 9 Baby&Thresia, Thanni, Mayyanad.P.O Mayyanadu. -

Annexure 1 B - Kollam
Annexure 1 B - Kollam Allotted Mobile Nos Sl.No Designation/Post Allotted Office District Allotted 1 Kollam 9383470770 PAO Kollam District Office Kollam 2 Kollam 9383470102 JDA PDATMA KLM ATMA KLM 3 Kollam 9383470208 AO KB Nedumpana Chathannoor Block 4 Kollam 9383470210 AO KB Kalluvathukkal Chathannoor Block 5 Kollam 9383470213 AO KB Chirakkara Chathannoor Block 6 Kollam 9383470215 AO KB Chathannoor Chathannoor Block 7 Kollam 9383470217 AO KB Adichanelloor Chathannoor Block 8 Kollam 9383470219 AO KB Poothakulam Chathannoor Block 9 Kollam 9383470224 AO KB Paravoor Chathannoor Block 10 Kollam 9383470225 AO KB Sasthamkotta Sasthamcotta Block 11 Kollam 9383470227 AO KB Kunnathur Sasthamcotta Block 12 Kollam 9383470229 AO KB Poruvazhy Sasthamcotta Block 13 Kollam 9383470231 AO KB Sooranadu North Sasthamcotta Block 14 Kollam 9383470233 AO KB Sooranadu South Sasthamcotta Block 15 Kollam 9383470236 AO KB Mynagapally Sasthamcotta Block 16 Kollam 9383470238 AO KB West Kallada Sasthamcotta Block 17 Kollam 9383470316 DD(WM) PAO KLM 18 Kollam 9383470317 DD (NWDPRA) PAO KLM 19 Kollam 9383470318 DD (C ) PAO KLM 20 Kollam 9383470319 DD (YP) PAO KLM 21 Kollam 9383470320 DD (E &T) PAO KLM 22 Kollam 9383470313 DD (H) PAO KLM 23 Kollam 9383470230 TA PAO KLM 24 Kollam 9383470330 APAO PAO KLM 25 Kollam 9383470240 ACO PAO KLM 26 Kollam 9383470347 AA PAO KLM 27 Kollam 9383470550 ADA (Marketing) PAO KLM 28 Kollam 9383470348 ASC DSTL KLM 29 Kollam 9383470338 AO DSTL KLM 30 Kollam 9383470339 ASC MSTL KLM 31 Kollam 9383470331 AO MSTL KLM 32 Kollam 9383470332 ADA -

Accused Persons Arrested in Kollam Rural District from 13.12.2020To19.12.2020
Accused Persons arrested in Kollam Rural district from 13.12.2020to19.12.2020 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 KULATHO ANI VILASAM 19-12-2020 ANILKUMA THANKAPP 38, 1333/2020 OPUZHA ASHOK BAILED BY 1 CHELAPPALLY ARIPPA at 18:00 R AN Male U/s 279 IPC (Kollam KUMAR POLICE KOTTUKKAL Hrs Rural) 2966/2020 Sreesailam, , Shappumukk 18-12-2020 KUNDARA Ramachanra 37, U/s 279 IPC Rajeev, SI of 2 Rahul Kareepra u at 19:40 (Kollam ARRESTED - n Male & 185 MV Police Kuzhimathikkadu Perumpuzha Hrs Rural) ACT CHARUVILA 2646/2020 19-12-2020 KADAKKA SI Praveen RADHAKRI 28, PUTHEN U/s 15(c) r/w BAILED BY 3 JEYESH KOTTUKKAL at 19:05 L (Kollam Kadakkal SHNAN Male VEEDU,VAYALA,K 63 of Abkari POLICE Hrs Rural) Police Station OTTUKKAL Act 2646/2020 SIDHIK 19-12-2020 KADAKKA SI Praveen 26, U/s 15(c) r/w BAILED BY 4 FIROS SAKARIYA MANSIL,KOTTUK KOTTUKKAL at 19:05 L (Kollam Kadakkal Male 63 of Abkari POLICE KAL Hrs Rural) Police Station Act MECHERY VEEDU 19-12-2020 ANCHAL SIVANKUTT RAGHAVA 57, R.O.JUNCTIO 2865/2020 SREEJU J S, SI 5 VAYYANAM at 14:10 (Kollam ARRESTED - Y N PILLAI Male N,ANCHAL U/s 283 IPC OF POLICE ITTIVA Hrs Rural) PONNU VILA KIZHAKKATHIL, 19-12-2020 SOORANA 33, CHAKKUVA 1877/2020 BAILED BY 6 SIDIQ BASHEER KAMPALADY at 19:10 DU (Kollam P SREEJITH Male LLY U/s 279 IPC POLICE MURI, Hrs Rural) PORUVAZHY -
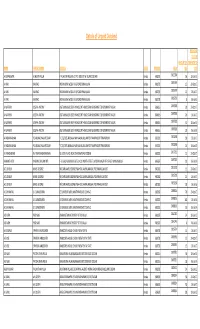
Details of Unpaid Dividend
Details of Unpaid Dividend PROPOSED DATE OF AMOUNT (IN TRANSFER TO NAME FATHER'S NAME ADDRESS STATE PINCODE FOLIO RS.) IEPF A GOPAKUMAR ACHUTAN PILLAI P. ACHUTAN PILLAI & CO P.O. BOX 507 W. ISLANO COCHIN Kerala 682003 0021306 56 18‐Jun‐15 A HARI KAK RAO INDIAN BANK WESDA. M G ROAD ERNAKULAM Kerala 682035 0039759 15 20‐Oct‐17 A HARI KAK RAO INDIAN BANK WESDA. M G ROAD ERNAKULAM Kerala 682035 0039759 15 16‐Jul‐17 A HARI KAK RAO INDIAN BANK WESDA. M G ROAD ERNAKULAM Kerala 682035 0039759 806‐Jul‐14 A MATHEW JOSEPH ANTONY ASST.MANAGER (EDP) FINANCE DPT HINDUSTAN NEWSPRINT LTD NEWSPRINT NAGAR Kerala 686616 0007828 20 20‐Oct‐17 A MATHEW JOSEPH ANTONY ASST.MANAGER (EDP) FINANCE DPT HINDUSTAN NEWSPRINT LTD NEWSPRINT NAGAR Kerala 686616 0007828 20 16‐Jul‐17 A MATHEW JOSEPH ANTONY ASST.MANAGER (EDP) FINANCE DPT HINDUSTAN NEWSPRINT LTD NEWSPRINT NAGAR Kerala 686616 0007828 32 18‐Jun‐15 A MATHEW JOSEPH ANTONY ASST.MANAGER (EDP) FINANCE DPT HINDUSTAN NEWSPRINT LTD NEWSPRINT NAGAR Kerala 686616 0007828 10 06‐Jul‐14 A PADMANABHAN P S ARUNACHALA REDDIAR T C 25/1695 ARUNALAYAM MANJALIKULAM RD THAMPANOOR TRIVANDRUM Kerala 695001 0018268 30 16‐Jul‐17 A PADMANABHAN P S ARUNACHALA REDDIAR T C 25/1695 ARUNALAYAM MANJALIKULAM RD THAMPANOOR TRIVANDRUM Kerala 695001 0018268 24 18‐Jun‐15 A VARADARAJAN R V ANANTHANARAYANAN V1/1673 PALACE ROAD THEKKAMADAM COCHIN Kerala 682002 0017215 15 20‐Oct‐17 A ANANTH IYER K ARUNA CHALAM IYER F‐3 GURU VAISHNAV FLATS 1‐A/10 NORTH STREET LAKSHMI NAGAR 4TH STAGE NANGANALLUR Kerala 695607 0007320 10 06‐Jul‐14 A C LOVELIN JAMES GEORGE -

Lake Anasagar, Ajmer, Rajasthan, India
Evidence‐Based Holistic Restoration of Lake Anasagar, Ajmer, Rajasthan, India Deep Narayan Pandey1*, Brij Gopal2, K. C. Sharma3 1 Member Secretary, Rajasthan State Pollution Control Board, Jaipur – 302015; Email: [email protected] 2 Ex-Professor, Jawahar Lal Nehru University, New Delhi, currently at Centre for Inland Waters in South Asia, National Institute of Ecology, Jaipur, Rajasthan 302017; Email: [email protected] 3 Professor and Head, Department of Environmental Science Central University of Rajasthan, NH-8 Bandarsidri, Kishangarh – 305801 Ajmer, Rajasthan, Email: [email protected] Views expressed in this paper are those of the authors; they do not necessarily represent the views of RSPCB or the institutions to which authors belong. Rajasthan State Pollution Control Board 4-Jhalana Institutional Area Jaipur 302 004, Rajasthan, India www.rpcb.nic.in 1 CONTENTS 1. Introduction 3 2. Present status of Lake Anasagar 3 3. Multiple stressors degrading the Lake Anasagar 4 3.1. Disposal of raw sewage and municipal wastewater 5 3.2. Discharge of detergents 6 3.3. Discharge of residual pesticides and fertilizers 7 3.4. Sedimentation due to soil erosion 7 3.5. Challenges of land ownership and encroachment 8 4. Holistic restoration of Lake Anasagar 8 4.1. Waste and sewage management 9 4.2. Forest restoration in the watershed 11 4.3. Sequential restoration of vegetation in sand dunes 12 4.4. Management of urban green infrastructure 13 4.5. Periodic sediment removal from lake 14 4.6. Macrophyte restoration in littoral zone of lake 15 4.7. Recovery of costs and reinvestment in urban systems 16 4.8. -

0 Cover Page
Mahatma Gandhi University 0 CONTENTS Sl.No Schools/Departments Page 1 School of Behavioural Sciences 1 2 School of Biosciences 13 3 School of Chemical Sciences 42 4 School of Computer Sciences 79 5 School of Environmental Sciences 86 6 School of Gandhian Thought & Development Studies 116 7 School of Indian Legal Thought 121 8 School of International Relations & Politics 124 9 School of Letters 131 10 School of Management and Business Studies 139 11 School of Pedagogical Sciences 150 12 School of Physical Education and Sports Sciences 158 13 School of Pure and Applied Physics 161 14 School of Social Sciences 180 15 School of Tourism Studies 186 16 Department of Lifelong Learning and Extension 197 17 Department of Printing and Publishing 198 Sl. No International and Inter University Centres/Inter School Centres 1 International and Inter University Centre for Nano Science and Nanotechnology 201 2 Advanced Centre for Environmental Studies and Sustainable Development 233 3 Inter University Centre for Bio Medical Research& Super Speciality 258 4 Inter University Centre for Disability Studies 266 5 Inter University -Instrumentation Centre 269 6 Inter University Centre for Social Science Research and Extension 294 7 Advanced Molecular Materials Research Centre 296 8 Centre for High Performance Computing 298 9 Institute for Contemporary Chinese Studies 300 10 Institute for Intensive Research in Basic Sciences 304 11 KN Raj Centre for Planning and Centre State Financial Relations 306 12 National Institute for Plant Science and Technology 310 13 University Centre for International Co-operation 316 Sl. No Constituent Colleges/Institution 1 University College of Engineering, Thodupuzha 319 2 School of Technology and Applied Sciences 323 3 School of Applied Life Sciences, Chuttippara 329 4 University Colleges of Teacher Education 331 5 Department of Library and Information Sciences 333 Publication details of Schools, Departments SCHOOL OF BEHAVIOURAL SCIENCES 3.4.2 Details of publications by the faculty Sl. -

Final Report
1 REPORT ON CRZ VIOLATIONS IN KOLLAM DISTRICT 1. INTRODUCTION As per the direction of Supreme Court to prepare the list of violations against CRZ Notification across the State, Government of Kerala vide Order No G.O (Rt) No 98/2017/Envt. dated Thiruvananthapuram, 16/10/2019 (Annexure 1) have constituted Coastal District Committees (CDC) for ten coastal districts including the district of Kollam with District Collector as Chairman and District Town Planner as Convener for preparing the list of violations against CRZ Notification. All the concerned local body secretaries and Village officers are the members. Accordingly, the first meeting of CDC, Kollam was convened on 24.10.2019 and an Action Plan for collection and compilation of list of CRZ violations was discussed and decided. (Minutes of first meeting is enclosed as Annexure II.) As neither guidelines nor prescribed formats for the collection of list of violations against CRZ Notification were provided, it is decided in the first CDC meeting to collect the details in two phases. In the first phase, the focus was to collect location wise (i.e., survey number wise) number of CRZ violations in every village included in CRZ Notification. Category of violations such as residential, commercial etc. and land development were also to be identified. In the second phase details such as name and address of owners, status of owners, distance to the violations from HTL, area of construction/land etc. of identified CRZ violations were to be collected. Accordingly, Ist,, IInd and IIIrd Interim Report on CRZ violations were prepared based on the formats (Annexure III) issued to all concerned local bodies and the reports were submitted to the Chief Secretary to Government on due dates viz.31-10-19, 30-11-19 and 20-12-19 respectively. -

Ashtamudi Lake - an Overview on Physical Characteristics
Vol 11, Issue 6,June/ 2020 ISSN NO: 0377-9254 ASHTAMUDI LAKE - AN OVERVIEW ON PHYSICAL CHARACTERISTICS Saranya U* and Prof. (Dr.) Lancelet T.S** *Research Scholar, Dept. of Geography, SSUS, Kalady **Professor, Dept. of Geography, SSUS, Kalady e-mail - [email protected], [email protected] Abstract Water is one of the most important resources available on this earth that has an influence on Water is one of the most important resources human activities. All important aspects such as available on this earth that has an influence on agriculture, industry, day to day life is dependent human activities. The physical characteristics of on water. It is the most important factor for a place make up its natural environment and are survival of life on this earth. The physical derived from geological, hydrological, characteristics of a place make up its natural atmospheric, and biological processes. Surface environment and are derived from geological, and ground water are the important source of hydrological, atmospheric, and biological water on the earth where contemporary needs processes. They include land forms, bodies of focus on surface water and it leads to global water, climate, soils, natural vegetation and deterioration. Ashtamudi Lake is a unique water animal life (The Five Themes of Geography, system with limnological and hydrological AAG, 1984). Surface and ground water are the characteristics. Ashtamudi Lake derives its name important source of water on the earth where from its shape, having eight prominent arms or contemporary needs focus on surface water and channels branching out, like that of a palm tree it leads to global deterioration. -

Tears for a Once-Large Lake
Tears for a once-large lake To rephrase former British Prime Minister Winston Churchill, our public plan implementations are a mockery wrapped in sham, inside a farce. Sasthamkotta Lake, the state’s largest freshwater lake and one of the 26 Ramsar sites in the country, in Kollam district is a wasteland today. A Ramsar site is UN-speak for an internationally designated wetland site that should be conserved and sustainably developed and used. The deterioration that the lake has faced is the opposite of what should happen at a Ramsar site and the issue has been raised continually by local people and environmental groups. It looked like a real difference could be made when Oommen Chandy’s UDF government allocated Rs 24.8 crore for conservation and protection of the Sasthamkotta freshwater lake. Together with this, another over Rs 30 crore was allocated for the water needs of Kollam via Kallada river and Pallikal river. This was in June 2013. Things changed, but for the worse. The largest freshwater lake continued to shrink and encroachers are taking over the land that lies bare. The lakes natural aquifers are blocked with waste. So, our politicians have stepped in again with what they feel is the best remedy under the circumstances: forming a committee. The LDF government constituted an expert committee, who are expected to prepare a management action plan for the conservation and protection of the Lake. According a report in The Hindu (April 19), the new committee comprises ‘experts and bureaucrats connected with the subject’ and 12 special invitees, who are bureaucrats from various departments. -

Annual Report 2013 - 2014
ANNUAL REPORT 2013 - 2014 KERALA STATE BIODIVERSITY BOARD KSBB ANNUAL REPORT 2013 - 2014 Published by: Dr. K. P. Laladhas Member Secretary KERALA STATE BIODIVERSITY BOARD L-14, Jai Nagar, Medical College P.O., Thiruvananthapuram Ph: 0471-2554740, Telefax: 0471-2448234 Email: [email protected] Website: www.keralabiodiversity.org Design and layout www.communiquetvm.tumblr.com Cover Photo Gokul R. Purple Moorhen Photo: Riyas Arun T. KERALA STATE BIODIVERSITY BOARD 53 KERALA STATE BIODIVERSITY BOARD ANNUAL REPORT 2 0 1 3 - 2 0 1 4 L14, Jainagar, Medical College.P.O Thiruvananthapuram - 695 011 KERALA STATE BIODIVERSITY BOARD 1 2 KERALA STATE BIODIVERSITY BOARD C O N T E N T S 7 DOCUMENTATION OF BIODIVERSITY THROUGH PEOPLE’S BIODIVERSITY REGISTER (PBR) 9 MARINE BIODIVERSITY REGISTER (MBR) 10 STRENGTHENING BMC 12 CONSERVATION PROGRAMMES 21 BIODIVERSITY RESEARCH CENTRE 22 REGULATIONS AND NOTIFICATIONS 23 POLICY ISSUES AND ADVICE TO GOVERNMENT 26 NATURE EDUCATION 29 BIODIVERSITY AWARENESS PROGRAMMES 31 WORKSHOP/ SEMINARS 34 AWARDS/ RECOGNITIONS 37 PUBLICATIONS 40 REPRESENTATION IN EXPERT COMMITTEES AND OUTREACH PROGRAMMES 43 FUTURE PLANS 45 AUDITED STATEMENTS OF ACCOUNTS FOR THE YEAR 2013-14 KERALA STATE BIODIVERSITY BOARD 3 Dr.Oommen.V.Oommen Chairman Prelude In October 2010 in Nagoya, Japan, over 190 countries around the world reached a historic global agreement to take urgent action to halt the loss of biodiversity. The international community is eagerly looking out for the 12th meeting of the Conference of the Parties to the Convention on Biological Diversity with focus on sustainable development from 6 - 17 October 2014 at Pyeongchang, South Korea. This is the time for reckoning by all committed to safeguarding the variety of life on Earth.