Lateral Periodontal Cyst Treated with Enucleation and Guided Bone Regeneration: a Report of a Case and a Review of Pertinent Literature
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary for Narrative Writing
Periodontal Assessment and Treatment Planning Gingival description Color: o pink o erythematous o cyanotic o racial pigmentation o metallic pigmentation o uniformity Contour: o recession o clefts o enlarged papillae o cratered papillae o blunted papillae o highly rolled o bulbous o knife-edged o scalloped o stippled Consistency: o firm o edematous o hyperplastic o fibrotic Band of gingiva: o amount o quality o location o treatability Bleeding tendency: o sulcus base, lining o gingival margins Suppuration Sinus tract formation Pocket depths Pseudopockets Frena Pain Other pathology Dental Description Defective restorations: o overhangs o open contacts o poor contours Fractured cusps 1 ww.links2success.biz [email protected] 914-303-6464 Caries Deposits: o Type . plaque . calculus . stain . matera alba o Location . supragingival . subgingival o Severity . mild . moderate . severe Wear facets Percussion sensitivity Tooth vitality Attrition, erosion, abrasion Occlusal plane level Occlusion findings Furcations Mobility Fremitus Radiographic findings Film dates Crown:root ratio Amount of bone loss o horizontal; vertical o localized; generalized Root length and shape Overhangs Bulbous crowns Fenestrations Dehiscences Tooth resorption Retained root tips Impacted teeth Root proximities Tilted teeth Radiolucencies/opacities Etiologic factors Local: o plaque o calculus o overhangs 2 ww.links2success.biz [email protected] 914-303-6464 o orthodontic apparatus o open margins o open contacts o improper -

Lateral Periodontal Cysts: a Retrospective Study of 11 Cases
Med Oral Patol Oral Cir Bucal. 2008 May1;13(5):E313-7. Lateral periodontal cyst Med Oral Patol Oral Cir Bucal. 2008 May1;13(5):E313-7. Lateral periodontal cyst Lateral periodontal cysts: A retrospective study of 11 cases María Florencia Formoso Senande 1, Rui Figueiredo 2, Leonardo Berini Aytés 3, Cosme Gay Escoda 4 (1) Resident of the Master of Oral Surgery and Implantology. University of Barcelona Dental School (2) Associate Professor of Oral Surgery. Professor of the Master of Oral Surgery and Implantology. University of Barcelona Dental School (3) Professor of Oral Surgery. Professor of the Master of Oral Surgery and Implantology. Dean of the University of Barcelona Dental School (4) Chairman of Oral and Maxillofacial Surgery. Director of the Master of Oral Surgery and Implantology. University of Barcelona Dental School. Oral and maxillofacial surgeon of the Teknon Medical Center, Barcelona (Spain) Correspondence: Prof. Cosme Gay Escoda Centro Médico Teknon C/ Vilana 12 08022 – Barcelona (Spain) E-mail: [email protected] Formoso-Senande MF, Figueiredo R, Berini-Aytés L, Gay-Escoda C. Received: 20/04/2007 Lateral periodontal cysts: A retrospective study of 11 cases. Med Oral Accepted: 29/03/2008 Patol Oral Cir Bucal. 2008 May1;13(5):E313-7. © Medicina Oral S. L. C.I.F. B 96689336 - ISSN 1698-6946 http://www.medicinaoral.com/medoralfree01/v13i5/medoralv13i5p313.pdf Indexed in: -Index Medicus / MEDLINE / PubMed -EMBASE, Excerpta Medica -SCOPUS -Indice Médico Español -IBECS Abstract Objective: To describe the clinical, radiological and histopathological features of lateral periodontal cysts among patients diagnosed in different centers (Vall d’Hebron General Hospital, Granollers General Hospital, the Teknon Medical Center, and the Master of Oral Surgery and Implantology of the University of Barcelona Dental School; Barcelona, Spain). -

Atypical Presentation of Lateral Periodontal Cyst in an Elderly Female Patient – a Rare Case Report
Journal of Dentistry Indonesia 2016, Vol. 23, No.1, xx-xx doi:10.14693/jdi.v23i1.xxx Journal of Dentistry Indonesia 2016, Vol. 23, No.1, 25-27 doi:10.14693/jdi.v23i1.967 CASE REPORT Atypical Presentation of Lateral Periodontal Cyst in an Elderly Female Patient – A Rare Case Report Renita Lorina Castelino, Kumuda Rao, Supriya Bhat, Subhas Gogineni Babu Department of Oral Medicine and Radiology, A B Shetty Memorial Institute of Dental Sciences, Nitte University, Mangalore 575018, India Correspondence e-mail to: [email protected] ABSTRACT The lateral periodontal lateral cyst (LPC) is an uncommon developmental odontogenic cyst defined as a radiolucent lesion which develops along the lateral aspect of an erupted vital tooth. LPC represents approximately 0.8% to 2% of all odontogenic cysts. The most frequently reported location of a lateral periodontal cyst is the mandibular canine- premolar area, followed by the anterior region of the maxilla. The lateral periodontal cyst is usually asymptomatic and presents as a round, oval or teardrop-like well-circumscribed inter-radicular radiolucent area, usually with a sclerotic margin lying between the apex and cervical margin of the teeth. The lateral periodontal cyst usually is seen in the fifth to sixth decade of life with a male preponderance. This paper reports an atypical case of an inter-radicular radiolucent cystic lesion in located between the mandibular central incisor and the canine area in an 87-year-old female patient mimicking clinically and radiographically as a residual cyst -

A New Approach for the Treatment of Lateral Periodontal Cysts with an 810-Nm Diode Laser
e120 A New Approach for the Treatment of Lateral Periodontal Cysts with an 810-nm Diode Laser Gaetano Isola, DDS, PhD, PG Oral Surg1 A lateral periodontal cyst (LPC) is Giovanni Matarese, DDS2/Giuseppe Lo Giudice, MD, DDS3 a rare but well-recognized type of Francesco Briguglio, DDS, PhD4/Angela Alibrandi, MD5 epithelial developmental odonto- Andrea Crupi, DDS, PhD4/Giancarlo Cordasco, MD, DDS6 genic cyst and has a prevalence of 7 Luca Ramaglia, DDS, PhD 1.5% among cysts of the jaw.1 LPCs are defined as radiolucent lesions The aim of this study was to test whether the combination of diode laser therapy that grow along the lateral surface and surgical treatment for a lateral periodontal cyst (LPC) would result in greater of an erupted vital tooth in which clinical improvement compared with surgery alone. A total of 18 patients with an inflammatory etiology has been LPCs were assessed for eligibility for this study. At baseline, each patient was excluded based on clinical and his- randomly allocated to one of two regimens: diode laser plus surgery (test group) 2 or traditional surgical treatment alone (control group). Healing parameters were tologic features. It has been hy- assessed at 7 to 21 days to monitor short-term complications, and periodontal pothesized that LPCs arise from the parameters were assessed at 3, 6, and 12 months to evaluate long-term healing. reduced enamel epithelium or the The test group demonstrated highly significant differences in both the short- epithelial rests of Malassez in the term and long-term parameters compared with the control group. -
![Odontogenic Cysts II [PDF]](https://docslib.b-cdn.net/cover/6217/odontogenic-cysts-ii-pdf-1046217.webp)
Odontogenic Cysts II [PDF]
Odontogenic cysts II Prof. Shaleen Chandra 1 • Classification • Historical aspects • Odontogenic keratocyst • Gingival cyst of infants & mid palatal cysts • Gingival cyst of adults • Lateral periodontal cyst • Botroyoid odontogenic cyst • Galandular odontogenic cyst Prof. Shaleen Chandra 2 • Dentigerous cyst • Eruption cyst • COC • Radicular cyst • Paradental cyst • Mandibular infected buccal cyst • Cystic fluid and its role in diagnosis Prof. Shaleen Chandra 3 Gingival cyst and midpalatal cyst of infants Prof. Shaleen Chandra 4 Clinical features • Frequently seen in new born infants • Rare after 3 months of age • Undergo involution and disappear • Rupture through the surface epithelium and exfoliate • Along the mid palatine raphe Epstein’s pearls • Buccal or lingual aspect of dental ridges Bohn’s nodules Prof. Shaleen Chandra 5 • 2-3 mm in diameter • White or cream coloured • Single or multiple (usually 5 or 6) Prof. Shaleen Chandra 6 Pathogenesis Gingival cyst of infants • Arise from epithelial remnants of dental lamina (cell rests of Serre) • These rests have the capacity to proliferate, keratinize and form small cysts Prof. Shaleen Chandra 7 Midpalatal raphe cyst • Arise from epithelial inclusions along the line of fusion of palatal folds and the nasal process • Usually atrophy and get resorbed after birth • May persist to form keratin filled cysts Prof. Shaleen Chandra 8 Histopathology • Round or ovoid • Smooth or undulating outline • Thin lining of stratified squamous epithelium with parakeratotic surface • Cyst cavity filled with keratin (concentric laminations with flat nuclei) • Flat basal cells • Epithelium lined clefts between cyst and oral epithelium • Oral epithelium may be atrpohic Prof. Shaleen Chandra 9 Gingival cyst of adults Prof. Shaleen Chandra 10 Clinical features • Frequency • 0.5% • May be higher as all cases may not be submitted to histopathological examination • Age • 5th and 6th decade • Sex • No predilection • Site • Much more frequent in mandible • Premolar-canine region Prof. -

Gingival Cyst of Adults- Two Case Reports and Literature Review
https://doi.org/10.5272/jimab.2018242.2065 Journal of IMAB Journal of IMAB - Annual Proceeding (Scientific Papers). 2018 Apr-Jun;24(2) ISSN: 1312-773X https://www.journal-imab-bg.org Case reports GINGIVAL CYST OF ADULTS- TWO CASE REPORTS AND LITERATURE REVIEW Elitsa Deliverska1, Aleksandar Stamatoski2 1) Department of Oral and Maxillofacial Surgery, Faculty of Dental Medicine, Medical University – Sofia, Bulgaria. 2) Department of maxillofacial surgery, Faculty of Dental Medicine, Ss. Cyril and Methodius University- Skopje, Macedonia. ABSTRACT usually found in the incisor, canine, and premolar areas. Background: Gingival cyst of adult is an [1, 3, 4] uncommon, small, non inflammatory, extra-osseous, Clinically, the gingival cysts may certainly occur developmental cyst of gingiva arising from the rests of without bone involvement and may appear as painless, dental lamina. small sessile soft tissue swellings, usually involving the Purpose: The aim of our paper is to present two rare interdental area of the attached gingiva. clinical cases of gingival cyst of adult. These lesions measure about 0.5 to 1 cm in diameter. Material and methods: In the present cases, the They are often bluish or blue-gray due to thinning of the combined anatomic characteristics of the soft tissue overlying mucosa. In some instances, the cyst may cause presentation and the osseous defect suggest that the lesion slight erosion of the surface of the bone, which is usually is a gingival cyst of adult. Two cases of gingival cyst were not detected on a radiograph but is apparent during surgical diagnosed and treated with exicisional biopsy followed by exploration. -

Self-Study Course Three Course
2014 self-study course three course The Ohio State University College of Dentistry is a recognized provider for ADA, CERP, and AGD Fellowship, Mastership and Maintenance credit. ADA CERP is a service of the American Dental Association to assist dental professionals in identifying quality providers of continuing dental education. ADA CERP does not approve or endorse individual courses or instructors, nor does it imply acceptance of credit hours by boards of dentistry. Concerns or complaints about a CE provider may be directed to the provider or to ADA CERP at www.ada.org/goto/cerp. The Ohio State University College of Dentistry is approved by the Ohio State Dental Board as a permanent sponsor of continuing dental education ABOUT this FREQUENTLY asked COURSE… QUESTIONS… Q: Who can earn FREE CE credits? . READ the MATERIALS. Read and review the course materials. A: EVERYONE - All dental professionals in your office may earn free CE contact . COMPLETE the TEST. Answer the credits. Each person must read the eight question test. A total of 6/8 course materials and submit an questions must be answered correctly online answer form independently. for credit. us . SUBMIT the ANSWER FORM Q: What if I did not receive a ONLINE. You MUST submit your confirmation ID? answers ONLINE at: A: Once you have fully completed your p h o n e http://dent.osu.edu/sterilization/ce answer form and click “submit” you will be directed to a page with a . RECORD or PRINT THE 614-292-6737 unique confirmation ID. CONFIRMATION ID This unique ID is displayed upon successful submission Q: Where can I find my SMS number? of your answer form. -

Oral Pathology Final Exam Review Table Tuanh Le & Enoch Ng, DDS
Oral Pathology Final Exam Review Table TuAnh Le & Enoch Ng, DDS 2014 Bump under tongue: cementoblastoma (50% 1st molar) Ranula (remove lesion and feeding gland) dermoid cyst (neoplasm from 3 germ layers) (surgical removal) cystic teratoma, cyst of blandin nuhn (surgical removal down to muscle, recurrence likely) Multilocular radiolucency: mucoepidermoid carcinoma cherubism ameloblastoma Bump anterior of palate: KOT minor salivary gland tumor odontogenic myxoma nasopalatine duct cyst (surgical removal, rare recurrence) torus palatinus Mixed radiolucencies: 4 P’s (excise for biopsy; curette vigorously!) calcifying odontogenic (Gorlin) cyst o Pyogenic granuloma (vascular; granulation tissue) periapical cemento-osseous dysplasia (nothing) o Peripheral giant cell granuloma (purple-blue lesions) florid cemento-osseous dysplasia (nothing) o Peripheral ossifying fibroma (bone, cartilage/ ossifying material) focal cemento-osseous dysplasia (biopsy then do nothing) o Peripheral fibroma (fibrous ct) Kertocystic Odontogenic Tumor (KOT): unique histology of cyst lining! (see histo notes below); 3 important things: (1) high Multiple bumps on skin: recurrence rate (2) highly aggressive (3) related to Gorlin syndrome Nevoid basal cell carcinoma (Gorlin syndrome) Hyperparathyroidism: excess PTH found via lab test Neurofibromatosis (see notes below) (refer to derm MD, tell family members) mucoepidermoid carcinoma (mixture of mucus-producing and squamous epidermoid cells; most common minor salivary Nevus gland tumor) (get it out!) -
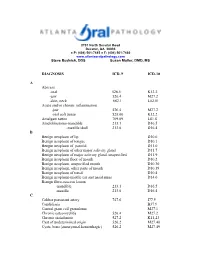
DIAGNOSIS ICD-9 ICD-10 a Abscess
2701 North Decatur Road Decatur, GA 30033 ● P: (404) 501-7445 ● F: (404) 501-7460 www.atlantaoralpathology.com Steve Budnick, DDS Susan Muller, DMD, MS DIAGNOSIS ICD-9 ICD-10 A Abscess -oral 528.5 K12.2 -jaw 526.4 M27.2 -skin, neck 682.1 L02.01 Acute and/or chronic inflammation -jaw 526.4 M27.2 -oral soft tissue 528.00 K12.2 Amalgam tattoo 709.09 L81.8 Ameloblastoma-mandible 213.1 D16.5 -maxilla/skull 213.0 D16.4 B Benign neoplasm of lip D10.0 Benign neoplasm of tongue D10.1 Bengin neoplasm of parotid D11.0 Benign neoplasm of other major salivary gland D11.7 Benign neoplasm of major salivary gland, unspecified D11.9 Benign neoplasm floor of mouth D10.2 Benign neoplasm, unspecified mouth D10.30 Benign neoplasm, other parts of mouth D10.39 Benign neoplasm of tonsil D10.4 Benign neoplasm-middle ear and nasal sinus D14.0 Benign fibro-osseous lesion -mandible 213.1 D16.5 -maxilla 213.0 D16.4 C Caliber persistent artery 747.6 I77.9 Candidiasis B37.9 Central giant cell granuloma M27.1 Chronic osteomyelitis 526.4 M27.2 Chronic sialadenitis 527.2 K11.23 Cyst of undetermined origin 526.2 M27.40 Cysts, bone{aneurysmal,hemorrhagic) 526.2 M27.49 D Dental follicle (enlarged) 526.9 M27.0 Dentigerous cyst 526.0 K09.0 Dental granuloma 526.4 M27.2 Developmental cyst NOS 526.1 K09.0 Dysplasia – mid 528.79 K13.29 -moderate 528.79 K13.29 -severe 528.79 K13.29 E Epidermoid cyst – mouth 528.4 K09.9 - skin 706.2 L72.0 Epulis fissuratum 528.9 K13.70 Eruption cyst 526.0 K09.0 Erythema migrans 529.1 K14.1 Erythema multiforme 695.1 L51.8 Exostosis 526.81 M27.8 F -

23. Lateral Periodontal Cyst- a Case Report
Sarkar S et al. Lateral Periodontal Cyst. Case Report LATERAL PERIODONTAL CYST: A CASE REPORT Subhadeep Sarkar 1, Obaid Khursheed 2, Ruby Bansal 1, Anuj Gaur 3 Departments of 1Oral and Maxillofacial Pathology, 2Pedodontics, 3Oral Medicine and Radiology, K.D. Dental College &Hospital, Mathura ABSTRAC T: The lateral periodontal cyst (LPC) is a harmless developmental aberration derived from pulp infection, infectionthrough the gingival crevice or odontogenic epithelia lying between the roots of vital teeth. The designation ‘lateral periodontal cyst’ is confined tothose cysts that occur in the lateral periodontal position. Standish and Shafer (1958) commented that the lateral periodontal cyst was of varied aetiology but that the term ‘lateral periodontal cyst’ should be used to indicate all cysts developing in the anatomical region of the lateral periodontium. The purpose of this article is to report a case of lateral periodontal cyst, presenting with typical clinical features, review the relevant literature which describes the etiopathogenesis, radiological histopathological features and successful surgical therapy of lateral periodontal cysts. Keywords- Lateral Periodontal Cyst, Etiopathogenesis of LPC, Odontogenic cysts. Corresponding Author: Dr. Subhadeep Sarkar, Department of Oral and Maxillofacial Pathology, K.D. Dental College &Hospital, Mathura-281003. E-mail: [email protected] This article may be cited as: Sarkar S, Khursheed O, Bansal R, Gaur A. Lateral Periodontal Cyst: A Case Report. J Adv Med Dent Scie Res 2015;3(2):119-122. INTRODUCTION Odontogenic cysts are classified by the World Health mandible, followed by the anterior segment of the Organization as inflammatory and developmental maxillary alveolar process. LPC arise preferentially according to their epithelial lining. -

DH 318 General and Oral Pathology
DH 248 General and Oral Pathology Spring 2014 Meeting Times: Tuesday & Thursday 10:00 - 11:50 a.m. CASA Mortuary Science Room 70 Credits: 4 credit hours Faculty: Sherri Lukes, RDH, MS, Associate Professor, Room 129 Office: 453-7289 Cell: 521-3392 E-mail: [email protected] Office Hours: Monday 1:00 p.m. - 4:00 p.m. Tuesday 1:00-4:00 Other office hours by appointment COURSE DESCRIPTION: This course has been designed to integrate oral pathology and general pathology. Students will study principles of general pathology with emphasis on the relationships to oral diseases. Pathologic physiology is included such as tissue regeneration, the inflammatory process, immunology and wound healing. Clinical appearance, etiology, location and treatment options of general system diseases is presented, along with the oral manifestations. Special attention will be placed on common pathological conditions of the oral cavity and early recognition of these conditions. DH Competencies addressed in the course: PC.1 Systematically collect analyze, and record data on the general, oral, and psychosocial health status of a variety of patients/clients using methods consistent with medico-legal principles. PC.2 Use critical decision making skills to reach conclusions about the patient’s/client’s dental hygiene needs based on all available assessment data. PC.3 Collaborate with the patient / client, and/or other health professionals, to formulate a com- prehensive dental hygiene care plan that is patient / client-centered and based on current scientific evidence. PC.4 Provide specialized treatment that includes preventive and therapeutic services designed to achieve and maintain oral health. Assist in achieving oral health goals formulated in collaboration with the patient / client. -

Plain Xray Imaging – Principles, Patology
Radiology for stomatologists – plain Xray imaging – principles, patology Jakub stulík, prof. Karel Benda, Petr Nádeníček X-ray - attributes • Electromagnetic radiation of short wavelength produced when high-speed electrons strike a solid target • Ability to pass through tissues where is partially absorbed Radiolucency Radio-opacity (dark) (light) Plain X ray imaging • 1) Imaging of skull – basic projections • 2) Dental radiographs – A) Intraoral imaging – B) Extraoral imaging 1) Skull skiagrams - basic projections – Picture of the cranium – traumatic – Projection of paranasal sinuses – Orbits – Os temporale – Temporo-Mandibular Joint Cranium – dorso-ventral and lateral projection • Nose and forehead touch the cassette • X-ray pass through the protuber. occipitalis perpendicularly to cassete Cranium – dorso-ventral and lateral projection Cranium – lateral projection • Central beam goes through the acustic meatus • Perpendicular to the cassette Cranium – lateral projection Paranasal sinuses – Water´s projection orbito-meatal line Orbito-meatal Line Orbits – dorso-ventral projection Orbits – lateral projection Skeleton Points Soft Tissue Points 14 sella Os temporale – Stenver´s – semisagital pr. • http://rtg.misto.cz/_MAIL_/hlava/11.jpg Os temporale – Schüller´s – semilateral • http://rtg.misto.cz/_MAIL_/hlava/12.jpg projection Os temporale – Schüller´s – semilateral projection Temporomandibular joint (TMJ) • Intracapsul. dissease = diskopathy- we can see calcifications • Correct position of temporo mandible joint (TMJ) Temporomandibular joint - TMJ serial radiogram TMJ • condyl head • x-ray beam pass vertical +25° to center of film • fossa glenoidalis • entering 6-7cm over meatus acusticus. • close mouth • open mouth 2a) Intraoral exposures Intraoral X-ray device • voltage of X-ray tube – 50-90 kV • filtration of primary beam – 1,5 mm Al - U<70 kV – 2,5 mm Al - U > 70 kV • body tube – length of body tube = 10-30 cm RADIATION PROTECTION • Use of proper exposure and processing techniques • Patients should be shielded with lead aprons and thyroid shields.