4.1.5 Quadratus Lumborum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

University Microfilms 300 North 2Eeb Road Ann Arbor, Michigan 48106
INFORMATION TO USERS This dissertation was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns ...tch may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)''. If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. You will find a good image of the page in the adjacent frame. 3. When a map, drawing or chart, etc., was part of the material being photographed the photographer followed a definite method in "sectioning" the material. It is customary to begin photoing at the upper left hand corner of a large sheet and to continue photoing from left to right in equal sections with a small overlap. If necessary, sectioning is continued again — beginning below the first row and continuing on until complete. 4. The majority of users indicate that the textual content is of greatest value, however, a somewhat higher quality reproduction could be made from "photographs" if essential to the understanding of the dissertation. -

Transabdominal Extraperitoneal Section of the Obturator Nerve Trunk Paul H
TRANSABDOMINAL EXTRAPERITONEAL SECTION OF THE OBTURATOR NERVE TRUNK PAUL H. HARMON, M.D. Department of Orthopedic Surgery, Permanente Hospitals and The Permanente Foundation, Oakland, California (Received for publication September 8, 1949) POPULAR method of interrupting section of the obturator nerve is to section its many peripheral branches high in the medial thigh as A originally described by Stoffel 6,7 in 1910. However, obturator nerve section in the thigh is frequently not as effective as section of the trunk higher because of accessory obturator nerves and branches of the main obturator trunk which may originate within the abdomen and pursue a variable peripheral course. Selig4'~ in 1913 and 1914 reported an anatomical study demonstrating the possibility of low intrapelvic extraperitoneal section of the obturator trunk. A number of authors (reviewed by Chandler and Seidler2 and by Wis- chnewsky s) have reported on the use of this technique. Chandler and Seidler2 reported 84 eases in 1939, in which the nerve was approached through a lower abdominal incision, just lateral to the lower border of the rectus muscle. In cases of bilateral section of the nerve these authors made a trans- verse skin incision with vertical deep dissection on the lateral side of each rectus abdominis muscle. Bonne0 described a lateral iliolumbar approach through which the obturator nerve was located high beneath the iliopsoas muscle. The disadvantage of this technique is the lengthy incision and deep dissection. Recently, Freeman 3 reported the combined section of the obtu- rator and femoral nerves in paraplegics, through a single vertical incision which crossed Poupart's ligament. -

Familial Absenceof the Pectoralis Major, Serratus Anterior, And
J Med Genet: first published as 10.1136/jmg.22.5.390 on 1 October 1985. Downloaded from Journal of Medical Genetics, 1985, 22, 390-392 Familial absence of the pectoralis major, serratus anterior, and latissimus dorsi muscles T J DAVID* AND R M WINTERt From *the Department of Child Health, University ofManchester; and tthe Kennedy-Galton Centrefor Clinical Genetics, Harperbury Hospital, Hertfordshire. SUMMARY Congenital absence of shoulder girdle muscles is described in three generations of a family. The proband, a 3 year old boy, had absence of the sternocostal head of the right pectoralis major. His father had absence of the left serratus anterior and part of the left latissimus dorsi and his paternal grandfather had absence of the lower two-thirds of the left pectoralis major, with absence of the left serratus anterior and latissimus dorsi muscles. The condition is probably the result of a dominant gene. These observations show that absence of the pectoralis major is part of a wider spectrum of shoulder girdle defects. Where genetic advice is sought by persons with apparently sporadic absence of the pectoralis major, examination of the relatives is necessary. The Poland anomaly comprises unilateral absence of of the Poland anomaly or isolated absence of the the pectoralis major combined with an ipsilateral pectoralis. The pedigree is shown in fig 1. malformation of the hand which usually includes syndactyly. It is currently unclear whether isolated Case reports absence of the pectoralis major muscle and the CASE 1 Poland anomaly are part of the same spectrum of IV.3 was a male infant, the product of the first defects or are separate entities.1 Both are consis- 30 year old http://jmg.bmj.com/ tently unilateral and both are usually sporadic pregnancy of a 29 year old mother and 2 father, who had been married for seven years. -

Quantification of the Elastic Moduli of Lumbar Erector Spinae And
applied sciences Article Quantification of the Elastic Moduli of Lumbar Erector Spinae and Multifidus Muscles Using Shear-Wave Ultrasound Elastography Tae Hyun Lim 1, Deukhee Lee 2,3 , Olga Kim 1,3 and Song Joo Lee 1,3,* 1 Center for Bionics, Biomedical Research Institute, Korea Institute of Science and Technology, Seoul 02792, Korea; [email protected] (T.H.L.); [email protected] (O.K.) 2 Center for Healthcare Robotics, AI and Robot Institute, Korea Institute of Science and Technology, Seoul 02792, Korea; [email protected] 3 Division of Bio-Medical Science and Technology, KIST School, Korea University of Science and Technology, Seoul 02792, Korea * Correspondence: [email protected]; Tel.: +82-2-958-5645 Featured Application: The findings and approach can potentially be used to guide surgical train- ing and planning for developing a minimal-incision surgical procedure by considering surgical position-dependent muscle material properties. Abstract: Although spinal surgeries with minimal incisions and a minimal amount of X-ray exposure (MIMA) mostly occur in a prone posture on a Wilson table, the prone posture’s effects on spinal muscles have not been investigated. Thus, this study used ultrasound shear-wave elastography (SWE) to compare the material properties of the erector spinae and multifidus muscles when subjects lay on the Wilson table used for spinal surgery and the flat table as a control condition. Thirteen male subjects participated in the study. Using ultrasound SWE, the shear elastic moduli (SEM) of the Citation: Lim, T.H.; Lee, D.; Kim, O.; Lee, S.J. Quantification of the Elastic erector spinae and multifidus muscles were investigated. -

The Pyramidalis–Anterior Pubic Ligament–Adductor Longus Complex (PLAC) and Its Role with Adductor Injuries: a New Anatomical Concept
The pyramidalis-anterior pubic ligament-adductor longus complex (PLAC) and its role with adductor injuries a new anatomical concept Schilders, Ernest; Bharam, Srino; Golan, Elan; Dimitrakopoulou, Alexandra; Mitchell, Adam; Spaepen, Mattias; Beggs, Clive; Cooke, Carlton; Holmich, Per Published in: Knee Surgery, Sports Traumatology, Arthroscopy DOI: 10.1007/s00167-017-4688-2 Publication date: 2017 Document version Publisher's PDF, also known as Version of record Document license: CC BY Citation for published version (APA): Schilders, E., Bharam, S., Golan, E., Dimitrakopoulou, A., Mitchell, A., Spaepen, M., Beggs, C., Cooke, C., & Holmich, P. (2017). The pyramidalis-anterior pubic ligament-adductor longus complex (PLAC) and its role with adductor injuries: a new anatomical concept. Knee Surgery, Sports Traumatology, Arthroscopy, 25(12), 3969- 3977. https://doi.org/10.1007/s00167-017-4688-2 Download date: 03. okt.. 2021 Knee Surg Sports Traumatol Arthrosc DOI 10.1007/s00167-017-4688-2 HIP The pyramidalis–anterior pubic ligament–adductor longus complex (PLAC) and its role with adductor injuries: a new anatomical concept Ernest Schilders1,2,3 · Srino Bharam3,4 · Elan Golan5 · Alexandra Dimitrakopoulou2,6 · Adam Mitchell7 · Mattias Spaepen8 · Clive Beggs2 · Carlton Cooke9 · Per Holmich10,11 Received: 29 April 2017 / Accepted: 16 August 2017 © The Author(s) 2017. This article is an open access publication Abstract Results The pyramidalis is the only abdominal muscle Purpose Adductor longus injuries are complex. The anterior to the pubic bone and was found bilaterally in all confict between views in the recent literature and various specimens. It arises from the pubic crest and anterior pubic nineteenth-century anatomy books regarding symphyseal ligament and attaches to the linea alba on the medial border. -

Towards Defining Muscular Regions of Interest from Axial Magnetic Resonance Imaging with Anatomical Cross-Reference: Part II - Cervical Spine Musculature James M
Elliott et al. BMC Musculoskeletal Disorders (2018) 19:171 https://doi.org/10.1186/s12891-018-2074-y TECHNICAL ADVANCE Open Access Towards defining muscular regions of interest from axial magnetic resonance imaging with anatomical cross-reference: part II - cervical spine musculature James M. Elliott1,2,3* , Jon Cornwall4, Ewan Kennedy5, Rebecca Abbott2 and Rebecca J. Crawford6 Abstract Background: It has been suggested that the quantification of paravertebral muscle composition and morphology (e.g. size/shape/structure) with magnetic resonance imaging (MRI) has diagnostic, prognostic, and therapeutic potential in contributing to overall musculoskeletal health. If this is to be realised, then consensus towards standardised MRI methods for measuring muscular size/shape/structure are crucial to allow the translation of such measurements towards management of, and hopefully improved health for, those with some musculoskeletal conditions. Following on from an original paper detailing methods for measuring muscles traversing the lumbar spine, we propose new methods based on anatomical cross-reference that strive towards standardising MRI-based quantification of anterior and posterior cervical spine muscle composition. Methods: In this descriptive technical advance paper we expand our methods from the lumbar spine by providing a detailed examination of regional cervical spine muscle morphology, followed by a comprehensive description of the proposed technique defining muscle ROI from axial MRI. Cross-referencing cervical musculature and vertebral -
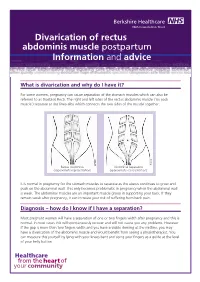
Divarication of Rectus Abdominis Muscle Postpartum Information And
Divarication of rectus abdominis muscle éçëíé~êíìã Information and advice What is divarication and why do I have it? For some women, pregnancy can cause separation of the stomach muscles which can also be referred to as Diastasis Recti. The right and left sides of the rectus abdominis muscle (‘six pack muscle’) separate at the linea alba which connects the two sides of the muscle together. Rectus Abdominis Abdominal Separation (approximate representation) (approximate representation) It is normal in pregnancy for the stomach muscles to separate as the uterus continues to grow and push on the abdominal wall. This only becomes problematic in pregnancy when the abdominal wall is weak. The abdominal muscles are an important muscle group in supporting your back. If they remain weak after pregnancy, it can increase your risk of suffering from back pain. Diagnosis – how do I know if I have a separation? Most pregnant women will have a separation of one or two fingers width after pregnancy and this is normal. In most cases this will spontaneously recover and will not cause you any problems. However if the gap is more than two fingers width and you have a visible doming at the midline, you may have a divarication of the abdominis muscle and would benefit from seeing a physiotherapist. You can measure this yourself by lying with your knees bent and using your fingers as a guide at the level of your belly button. What can I do to help myself? Try to avoid all activities which place a lot of pressure on your abdominal wall, or cause an over stretch to your stomach. -

Effect of Latissimus Dorsi Muscle Strengthening in Mechanical Low Back Pain
International Journal of Science and Research (IJSR) ISSN: 2319-7064 ResearchGate Impact Factor (2018): 0.28 | SJIF (2019): 7.583 Effect of Latissimus Dorsi Muscle Strengthening in Mechanical Low Back Pain 1 2 Vishakha Vishwakarma , Dr. P. R. Suresh 1, 2PCPS & RC, People’s University, Bhopal (M.P.), India Abstract: Mechanical low back pain (MLBP) is one of the most common musculoskeletal pain syndromes, affecting up to 80% of people at some point during their lifetime. Sources of back pain are numerous, usually sought in as lesion of disc or facet joints at L4- L5 and L5-S1 levels. Studies have shown that 40% of all back pain is of thoracolumbar origin. The term meachnical low back pain also gives reassurance that there is no damage to the nerves or spinal pathology. The clinical presentation of mechanical low back pain usually the ages 18-55 years is in the lumbo sacral region. A study was conducted to evaluate the designed to check the effectiveness of Conventional Exercises alone in Mechanical low back pain and along with the latissimus dorsi muscle strengthening, data was collected from People’s hospital, Bhopal (age group-30-45 yr both male and female randomly) Keywords: Visual analog scale, Assessment chart, Treatment table, Data collection sheets, Essential stationery materials, Computer, SPSS Software etc. 1. Introduction Back pain is a primary to seek medical advice considering 80% of people suffering from back pain. Mechanical low back pain is defined as a result of minor intervertebral dysfunction and referred pain in the low back The latissimus dorsi is a large, flat muscle on the back that and hip region, and can often be confused with the other stretches to the sides, behind the arm, and is partly covered pathologies that may cause these symptoms.18 by the trapezius on the back near the midline, the word latissimus dorsi comes from Latin and its means, broadest muscle of the back dorsum means back. -

Study of Axillary Arch: Embryological Basis and Its Clinical Implications
Original Article Indian Journal of Anatomy291 Volume 7 Number 3, May - June 2018 DOI: http://dx.doi.org/10.21088/ija.2320.0022.7318.12 Study of Axillary Arch: Embryological Basis and Its Clinical Implications Divya Shanthi D’Sa1, Vasudha T.K.2 Abstract Aim: To study the incidence, embryological basis and clinical implications of unusual but most common anatomical variant, axillary arch. Materials & Methods: The study was conducted in the department of Anatomy, Subbaiah Institute of Medical Sciences, Shimoga during routine cadaveric dissection on 60 upper limbs. Results: Of the 60 upper limbs dissected, Axillary arch was seen in one right upper limb. It was extending between latissimus dorsi and fascia covering the subscapularis muscle after crossing the posterior cord of brachial plexus and circumflex scapular vessels. It was supplied by a branch from the thoracodorsal nerve. Conclusion: The presence of an axillary arch needs to be considered in differential diagnosis of axillary swellings and also in surgeries of shoulder region. Therefore it is mandatory to know the variant slips of the musculotendinous arch. Keywords: Axillary Arch; Latissimus Dorsi; Neurovascular Bundle; Clinical Implications. Introduction other variants of this anomaly have also been observed like the muscle adhering to the coracoid process of the scapula, medial epicondyle of the Humerus or The axillary arch muscle is an accessory muscle blending with the fibers of teres major, long head of that extends between the pectoralis major and triceps brachii, coracobrachialis -

Differences in Electromyographic Activity in the Multifidus Muscle And
Eur Spine J (2002) 11:13–19 DOI 10.1007/s005860100314 ORIGINAL ARTICLE L. A. Danneels Differences in electromyographic activity P. L. Coorevits A. M. Cools in the multifidus muscle G. G. Vanderstraeten D. C. Cambier and the iliocostalis lumborum E. E. Witvrouw H. J. De Cuyper between healthy subjects and patients with sub-acute and chronic low back pain Abstract The present study was differences between patients and Received: 2 February 2001 Revised: 12 May 2001 carried out to examine possible controls were found for the nor- Accepted: 28 May 2001 mechanisms of back muscle dysfunc- malised EMG activity of the two Published online: 14 July 2001 tion by assessing a stabilising and a muscles. These findings indicated © Springer-Verlag 2001 torque-producing back muscle, the that, during low-load exercises, no multifidus (MF) and the iliocostalis insufficiencies in back muscle re- lumborum pars thoracis (ICLT), re- cruitment were evident in either sub- spectively, in order to identify acute or chronic back pain patients. whether back pain patients showed During the strength exercises, the altered recruitment patterns during normalised activity of both back different types of exercise. In a muscles was significantly lower group of healthy subjects (n=77) and in chronic low back pain patients patients with sub-acute (n=24) and (P=0.017 and 0.003 for the MF and chronic (51) low back pain, the nor- ICLT, respectively) than in healthy malised electromyographic (EMG) controls. Pain, pain avoidance and activity of the MF and the ICLT (as deconditioning may have contributed a percentage of maximal voluntary to these lower levels of EMG activ- contraction) were analysed during ity during intensive back muscle coordination, stabilisation and contraction. -

Lumbar Muscle Function and Dysfunction in Low Back Pain - Markku Kankaanpää
PHYSIOLOGY AND MAINTENANCE – Vol. IV - Lumbar Muscle Function and Dysfunction in Low Back Pain - Markku Kankaanpää LUMBAR MUSCLE FUNCTION AND DYSFUNCTION IN LOW BACK PAIN Markku Kankaanpää Department of Physical Medicine and Rehabilitation, Kuopio University Hospital, and Department of Physiology, University of Kuopio, Finland Keywords: Low back pain, trunk muscles, muscle coordination, dysfunction, biomechanics, deconditioning syndrome. Contents 1. Anatomy and Function of the Trunk Extensor and Flexor Muscles 1.1. Functional Properties of Lumbar Spine 1.2. Anatomy of Lumbar and Abdominal Muscles 1.3. Control Properties of Lumbar and Abdominal Muscles 2. Epidemiological Aspects of LBP 2.1. Physical Risk Factors of LBP 3. Structural and Pathophysiological Aspects in LBP 4. Lumbar Muscle Dysfunction in LBP 4.1. Loss of Strength 4.2. Excessive Lumbar Muscle Fatigue 4.3. Loss of Co-ordination and Muscle Control 4.4. Active Rehabilitation and Back Extensor Muscle Functional Assessment Glossary Bibliography Biographical Sketch Summary Low back pain is one of the most common health problems. The reason for back pain is most often unknown. Some of the models that help in explaining the origin of low back pain are introduced. The lumbar structure and muscle functions and dysfunctions in relation to low back pain are reviewed. Complex central and peripheral elements control the biomechanics of the lumbar spine and ensure optimal spinal loading in normal everyday life situations.UNESCO Low back pain leads to acute – and EOLSS chronic changes in paraspinal muscles and their control mechanisms. Acute changes are observed as impaired reflexive functions of paraspinal muscles. Pain in spinal structures results from reflexive activities that protect the spine from excessiveSAMPLE and harmful loading. -

Anatomy and Physiology of the Abdominal Wall 0011 CHAPTER Internal Oblique Some Inferior fi Bers Form the Cremaster Muscle at the Level of the Inguinal Canal
Handbook of Complex Abdominal Wall Anatomy and physiology of the abdominal wall 0011 CHAPTER Álvaro Robín Valle de Lersundi, MD, PhD Arturo Cruz Cidoncha, MD, PhD 11.1..1. Anatomy of the abdominal wall 1.1.1. Introduction The abdominal wall is delimited by muscle structures than can be classifi ed in 5 ana- tomical areas: lateral, anterior, posterior, diaphragmatic and perineal (Table 1.1). We will describe the fi rst four due to their relevance in surgical repair of complex abdom- inal wall. These groups of muscles are enclosed by several bone structures: last ribs, chondrocostal joints, xyphoid, pelvis and costal apophysis of lumbar vertebrae. Layers of the anterior and lateral abdominal wall include skin, subcutaneous tissue, super- fi cial fascia, deep fascia, muscles, extraperitoneal fascia and peritoneum. Table 1.1. Muscular limits of the abdominal wall ∙ Quadratus lumborum POSTERIOR ∙ Psoas ∙ Iliac muscle ∙ External oblique LATERAL ∙ Internal oblique ∙ Transversus abdominis ∙ Rectus abdominis ANTERIOR ∙ Piramidalis CONTENTS SUPERIOR ∙ Diaphragm 1.1. Anatomy of the abdominal INFERIOR ∙ Perineal muscles wall 1.1.2. Muscles of the abdominal wall 1.2. Physiologyygy Muscles of the anterolateral wall Rectus abdominis The rectus abdominis (m. rectus abdominis) is a long and thick muscle that is extended from the anterolateral thorax to the pubis close to the midline (Figure 1.1). 1 Figure External oblique 1.1. Rectus abdominis The external oblique muscle (m. obliquus externus abdominis) is the most superfi cial and thickest of the three lateral abdominal wall muscles (Figure 1.2). Figure 1.2. External oblique muscle Handbook of Complex Abdominal Wall Handbook of Complex Cranially, the rectus abdominis muscles originates from 3 dig- itations that insert on the 5th-7th costal cartilages, the xyphoid process and costoxyphoid ligament.