Association of Uranium with Iron Oxides Typically Formed On
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Extraction Processing of Concentrated Solutions of Uranyl Nitrate with High Impurities Content V.M
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Electronic archive of Tomsk Polytechnic University Сhemistry UDC 546.791.02.238:66.061.51 EXTRACTION PROCESSING OF CONCENTRATED SOLUTIONS OF URANYL NITRATE WITH HIGH IMPURITIES CONTENT V.M. Korotkevich, V.V.Lazarchuck, T.G. Shikerun, V.I. Shamin, N.A. Mikhailova, F.A. Dorda Federal Unitary Enterprise Siberian Group of Chemical Enterprises, Seversk Еmail: [email protected] Process flowsheet of recycling uranium concentrated solutions with its purification from insoluble impurities of iron, silicon, molybde num, calcium oxides and hydroxides and soluble impurities with application of centrifugal extractors cascade has been developed and suggested for commercial introduction. The process was carried out at extractant saturation (30 % tributyl phosphate in hydrocarbon diluent) in extraction assembly lower than a limiting level (85...95 g/l) and in wash assembly – at limiting saturation (up to 120 g/l). As a result the waste uranium content in watertail solutions 0,01...0,04 g/l and minimal content of impurities in reextractors is provided. Perspective program on development of nuclear technology are also: reliability of hermetization, possi power engineering proposes to involve wide range of raw bility of remote service, resistance to corrosive attack of materials from uranium ores to secondary uranium ma structural materials, duration of overhaul period etc. terials into processing at atomic enterprises. In this case In Russia the centrifugal extractor of ECТseries the problem of reprocessing concentrated uranium so with continuous extraction of solid phase [3], which may lutions with high impurity content occurs. -

Relationships Between Magnetic Parameters, Chemical Composition and Clay Minerals of Topsoils Near Coimbra, Central Portugal
Nat. Hazards Earth Syst. Sci., 12, 2545–2555, 2012 www.nat-hazards-earth-syst-sci.net/12/2545/2012/ Natural Hazards doi:10.5194/nhess-12-2545-2012 and Earth © Author(s) 2012. CC Attribution 3.0 License. System Sciences Relationships between magnetic parameters, chemical composition and clay minerals of topsoils near Coimbra, central Portugal A. M. Lourenc¸o1, F. Rocha2, and C. R. Gomes1 1Centre for Geophysics, Earth Sciences Dept., University of Coimbra, Largo Marquesˆ de Pombal, 3000-272 Coimbra, Portugal 2Geobiotec Centre, Geosciences Dept., University of Aveiro, 3810-193 Aveiro, Portugal Correspondence to: A. M. Lourenc¸o ([email protected]) Received: 6 September 2011 – Revised: 27 February 2012 – Accepted: 28 February 2012 – Published: 14 August 2012 Abstract. Magnetic measurements, mineralogical and geo- al., 1980). This methodology is fast, economic and can be ap- chemical studies were carried out on surface soil samples plied in various research fields, such as environmental mon- in order to find possible relationships and to obtain envi- itoring, pedology, paleoclimatology, limnology, archeology ronmental implications. The samples were taken over a and stratigraphy. Recent studies have demonstrated the ad- square grid (500 × 500 m) near the city of Coimbra, in cen- vantages and the potential of the environmental magnetism tral Portugal. Mass specific magnetic susceptibility ranges methods as valuable aids in the detection and delimitation between 12.50 and 710.11 × 10−8 m3 kg−1 and isothermal of areas affected by pollution (e.g. Bityukova et al., 1999; magnetic remanence at 1 tesla values range between 253 Boyko et al., 2004; Blaha et al., 2008; Lu et al., 2009; and 18 174 × 10−3 Am−1. -

Gas Phase Chemical Evolution of Uranium, Aluminum, and Iron Oxides Received: 22 January 2018 Batikan Koroglu1, Scott Wagnon 1, Zurong Dai1, Jonathan C
www.nature.com/scientificreports OPEN Gas Phase Chemical Evolution of Uranium, Aluminum, and Iron Oxides Received: 22 January 2018 Batikan Koroglu1, Scott Wagnon 1, Zurong Dai1, Jonathan C. Crowhurst1, Accepted: 19 June 2018 Michael R. Armstrong1, David Weisz1, Marco Mehl1,2, Joseph M. Zaug1, Harry B. Radousky1 & Published: xx xx xxxx Timothy P. Rose1 We use a recently developed plasma-fow reactor to experimentally investigate the formation of oxide nanoparticles from gas phase metal atoms during oxidation, homogeneous nucleation, condensation, and agglomeration processes. Gas phase uranium, aluminum, and iron atoms were cooled from 5000 K to 1000 K over short-time scales (∆t < 30 ms) at atmospheric pressures in the presence of excess oxygen. In-situ emission spectroscopy is used to measure the variation in monoxide/atomic emission intensity ratios as a function of temperature and oxygen fugacity. Condensed oxide nanoparticles are collected inside the reactor for ex-situ analyses using scanning and transmission electron microscopy (SEM, TEM) to determine their structural compositions and sizes. A chemical kinetics model is also developed to describe the gas phase reactions of iron and aluminum metals. The resulting sizes and forms of the crystalline nanoparticles (FeO-wustite, eta-Al2O3, UO2, and alpha-UO3) depend on the thermodynamic properties, kinetically-limited gas phase chemical reactions, and local redox conditions. This work shows the nucleation and growth of metal oxide particles in rapidly-cooling gas is closely coupled to the kinetically-controlled chemical pathways for vapor-phase oxide formation. Gas phase nucleation and growth of metal oxide nanoparticles is an important topic for many areas of chemistry, physics, material science, and engineering1–6. -

The Iron Oxides Structure, Properties, Reactions, Occurrence and Uses
R.M.Cornell U. Schwertmann The Iron Oxides Structure, Properties, Reactions, Occurrence and Uses Weinheim • New York VCH Basel • Cambridge • Tokyo Contents 1 Introduction to the iron oxides 1 2 Crystal structure 7 2.1 General 7 2.2 Iron oxide structures 7 2.2.1 Close packing of anion layers 10 2.2.2 Linkages of octahedra or tetrahedra 12 2.3 Structures of the individual iron oxides 14 2.3.1 The oxide hydroxides 14 2.3.1.1 Goethite a-FeOOH 14 2.3.1.2 Lepidocrocite y-FeO(OH) 16 2.3.1.3 Akaganeite ß-FeO(OH) and schwertmannite Fe16016(OH)y(S04)z • n H20 18 2.3.1.4 5-FeOOH and 8'-FeOOH (feroxyhyte) 20 2.3.1.5 High pressure FeOOH 21 2.3.1.6 Ferrihydrite 22 2.3.2 The hydroxides 24 2.3.2.1 Bernalite Fe(OH)3 • nH20 24 2.3.2.2 Fe(OH)2 25 2.3.2.3 Green rusts 25 2.3.3 The oxides 26 2.3.3.1 Haematite a-Fe203 26 2.3.3.2 Magnetite Fe304 28 2.3.3.3 Maghemite y-Fe203 30 2.3.3.4 Wüstite Fe^O 31 2.4 The Fe-Ti oxide System 33 3 Cation Substitution 35 3.1 General 35 3.2 Goethite 38 3.2.1 AI Substitution 38 3.2.1.1 Synthetic goethites 38 3.2.1.2 Natural goethites 43 3.2.2 Other substituting cations 43 3.3 Haematite 48 3.4 Other Fe oxides 50 VIII Contents 4 Crystal morphology and size 53 4.1 General 53 4.1.1 Crystal growth 53 4.1.2 Crystal morphology 55 4.1.3 Crystal size 57 4.2 The iron oxides 58 4.2.1 Goethite 59 4.2.1.1 General 59 4.2.1.2 Domainic character 64 4.2.1.3 Twinning 66 4.2.1.4 Effect of additives on goethite morphology 68 4.2.2 Lepidocrocite 70 4.2.3 Akaganeite and schwertmannite 71 4.2.4 Ferrihydrite 73 4.2.5 Haematite 74 4.2.6 Magnetite 82 4.2.7 -

Molecular Characterization of Uranium(VI) Sorption Complexes on Iron(III)-Rich Acid Mine Water Colloids
Geochimica et Cosmochimica Acta 70 (2006) 5469–5487 www.elsevier.com/locate/gca Molecular characterization of uranium(VI) sorption complexes on iron(III)-rich acid mine water colloids Kai-Uwe Ulrich a,*, Andre´ Rossberg a,b, Harald Foerstendorf a, Harald Za¨nker a, Andreas C. Scheinost a,b a Institute of Radiochemistry, FZ Rossendorf e.V., P.O. Box 510119, D-01314 Dresden, Germany b Rossendorf Beamline at ESRF, B.P. 220, F-38043 Grenoble, France Received 7 November 2005; accepted in revised form 21 August 2006 Abstract A mixing of metal-loaded acid mine drainage with shallow groundwater or surface waters usually initiates oxidation and/or hydrolysis of dissolved metals such as iron (Fe) and aluminum (Al). Colloidal particles may appear and agglomerate with increasing pH. Likewise chemical conditions may occur while flooding abandoned uranium mines. Here, the risk assessment of hazards requires reliable knowl- edge on the mobility of uranium (U). A flooding process was simulated at mesocosm scale by mixing U-contaminated acid mine water with near-neutral groundwater under oxic conditions. The mechanism of U-uptake by fresh precipitates and the molecular structure of U bonding were determined to estimate the mobility of U(VI). Analytical and spectroscopic methods such as Extended X-ray Absorption Fine-Structure (EXAFS) spectroscopy at the Fe K-edge and the U LIII-edge, and Attenuated Total Reflectance Fourier Transform Infra- red (ATR-FTIR) spectroscopy were employed. The freshly formed precipitate was identified as colloidal two-line ferrihydrite. It removed U(VI) from solution by sorption processes, while surface precipitation or structural incorporation of U was not observed. -

Depleted Uranium Technical Brief
Disclaimer - For assistance accessing this document or additional information,please contact [email protected]. Depleted Uranium Technical Brief United States Office of Air and Radiation EPA-402-R-06-011 Environmental Protection Agency Washington, DC 20460 December 2006 Depleted Uranium Technical Brief EPA 402-R-06-011 December 2006 Project Officer Brian Littleton U.S. Environmental Protection Agency Office of Radiation and Indoor Air Radiation Protection Division ii iii FOREWARD The Depleted Uranium Technical Brief is designed to convey available information and knowledge about depleted uranium to EPA Remedial Project Managers, On-Scene Coordinators, contractors, and other Agency managers involved with the remediation of sites contaminated with this material. It addresses relative questions regarding the chemical and radiological health concerns involved with depleted uranium in the environment. This technical brief was developed to address the common misconception that depleted uranium represents only a radiological health hazard. It provides accepted data and references to additional sources for both the radiological and chemical characteristics, health risk as well as references for both the monitoring and measurement and applicable treatment techniques for depleted uranium. Please Note: This document has been changed from the original publication dated December 2006. This version corrects references in Appendix 1 that improperly identified the content of Appendix 3 and Appendix 4. The document also clarifies the content of Appendix 4. iv Acknowledgments This technical bulletin is based, in part, on an engineering bulletin that was prepared by the U.S. Environmental Protection Agency, Office of Radiation and Indoor Air (ORIA), with the assistance of Trinity Engineering Associates, Inc. -
Nitrate (UO2 (NO)) 4
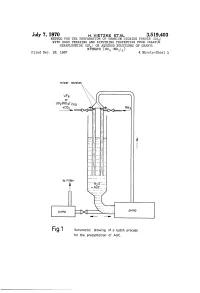
July 7, 1970 H. VETZKE ETA 3,519,403 METHOD FOR THE PREPARATION OF URANIUM DIOXIDE POWDER (UO) WITH GOOD PRESSING AND SINTERING PROPERTIES FROM URANIUM HEXAFLUORIDE (UF) OR AQUEOUS SOLUTIONS OF URANYL Fied Dec. 18, 1967 NiTRATE (UO2 (NO)) 4. Sheets-Sheet l mixer nozzles --race to fitter Fig.1 Schematic drawing of a batch process for the precipitation of AUC. July 7, 1970 H. VETZKE EA 3,519,403 METHOD FOR THE PREPARATION OF URANIUM DIOXIDE POWDER (UO) WITH GOOD PRESSING AND SINTERING PROPERTIES FROM RANIUM HEXAFLUORIDE (UF) OR AQUEOUS SOLUTIONS OF URANYL Filed Dec. 8, 1967 NiTRATE (UO2 (NO)) 4. Sheets-Sheet 3. mixer nozzles UF6 Of U02 (NO3)2ad tC92--X Ex-Ns / to filter cooling precipitation Vessel vessel Fig. 2 Schematic drawing of a continuous process for the precipitation of AUC July 7, 1970 H, VETZKE ETAL 3,519,403 METHOD FOR THE PREPARATION OF URANIUM DIOXIDE POWDER (UO) WITH GOOD PRESSING AND SINTERING PROPERTIES FROM URANIUM HEXAFLUORIDE (UF) OR AQUEOUS SOLUTIONS OF URANYL Filed Dec. 18, 1967 NiTRATE (UO (NO)2) 4. Sheets-Sheet 3 Off gas Off gas 487\m Nx UO2 powder XXX.& X.X S& steam / H2 Fig. 3 Ot Schematic drawing of a fluidized bed furnace for the reduction of AUC to U02, July 7, 1970 H. VEZKE ETAL 3,519,403 METHOD FOR THE PREPARATION OF URANIUM DIOXIDE POWDER (UO) WITH GOOD PRESSING AND SINTERING PROPERTIES FROM URANIUM HEXAFLUORIDE (UF) OR AQUEOUS SOLUTIONS OF URANYL NITRATE (UO2 (NO)a) Filed Dec. 18, 1967 4. Sheets-Sheet 1 -- Offgas Step 1: Decomposition, reduction and pyrohydrolysis i Step 2: Pyrohydrolysis Step 3: Controlled oxidation an o- are as an un- Fig. -

Thermal Route for the Synthesis of Maghemite/Hematite Core/Shell Nanowires
Thermal Route for the Synthesis of Maghemite/Hematite Core/Shell Nanowires Belén Cortés-Llanos,y,z,{ Aída Serrano,x,k Alvaro Muñoz-Noval,x,? Esteban Urones-Garrote,# Adolfo del Campo,k José F. Marco,@,{ Angel Ayuso-Sacido,z,4,r and Lucas Pérez∗,y,{,z yDept. Física de Materiales, Universidad Complutense de Madrid, 28040, Madrid, Spain zInstituto Madrileño de Estudios Avanzados - IMDEA Nanociencia, 28049, Madrid, Spain {Unidad Asociada IQFR (CSIC)-UCM, 28040, Madrid, Spain xSpLine, Spanish CRG BM25 Beamline, ESRF, 38000, Grenoble, France kInstituto de Cerámica y Vidrio, ICV-CSIC, 28049, Madrid, Spain ?Department of Applied Chemistry, Hiroshima University, Hiroshima 739-8527, Japan #Centro Nacional de Microscopía Electrónica, Universidad Complutense de Madrid, 28040, Madrid, Spain @Instituto de Química-Física Rocasolano CSIC, 28006, Madrid, Spain 4Fundacion de Investigacion HM Hospitales, 28050, Madrid, Spain rFacultad de Medicina (IMMA), Universidad San Pablo-CEU, 28925, Madrid, Spain E-mail: lucas.perez@fis.ucm.es Phone: +34 91 3944788 1 Abstract Nowadays, iron oxide-based nanostructures are key materials in many technological areas. Their physical and chemical properties can be tailored by tuning the morphology. In particular, the possibility of increasing the specific surface area has turned iron ox- ide nanowires (NWs) into promising functional materials in many applications. Among the different possible iron oxide NWs that can be fabricated, maghemite/hematite iron oxide core/shell have particular importance since they combine the magnetism of the inner maghemite core with the interesting properties of hematite in different techno- logical fields ranging from green energy to biomedical applications. However, the study of these iron oxide structures is normally difficult due to the structural and chemical similarities between both iron oxide polymorphs. -

Final Report
BNL-52661 Formal Report MECHANISMS OF RADIONUCLIDE-HYROXYCARBOXYLIC ACID INTERACTIONS FOR DECONTAMINATION OF METALLIC SURFACES A.J. FRANCIS, C.J. DODGE, AND J.B. GILLOW Brookhaven National Laboratory, Upton, NY 11973 G.P. HALADA AND C.R. CLAYTON Department of Materials Science, SUNY at Stony Brook, NY 11794 FINAL REPORT PROJECT PERIOD: 10/1/98 TO 9/30/01 PROJECT NUMBER: 64946 DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United State Government nor any agency thereof, nor any of their employees, not any of their contractors, subcontractors, or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency, contractor, or subcontractor thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency, contractor or subcontractor thereof. MECHANISMS OF RADIONUCLIDE-HYROXYCARBOXYLIC ACID INTERACTIONS FOR DECONTAMINATION OF METALLIC SURFACES* A.J. FRANCIS, C.J. DODGE, AND J.B. GILLOW Brookhaven National Laboratory, Upton, NY 11973 G.P. HALADA AND C.R. CLAYTON Department of Materials Science, SUNY at Stony Brook, NY 11794 FINAL REPORT PROJECT PERIOD: 10/1/98 TO 9/30/01 PROJECT NUMBER: 64946 *This work was performed under the auspices of the U.S. -

Inis: Terminology Charts
IAEA-INIS-13A(Rev.0) XA0400071 INIS: TERMINOLOGY CHARTS agree INTERNATIONAL ATOMIC ENERGY AGENCY, VIENNA, AUGUST 1970 INISs TERMINOLOGY CHARTS TABLE OF CONTENTS FOREWORD ... ......... *.* 1 PREFACE 2 INTRODUCTION ... .... *a ... oo 3 LIST OF SUBJECT FIELDS REPRESENTED BY THE CHARTS ........ 5 GENERAL DESCRIPTOR INDEX ................ 9*999.9o.ooo .... 7 FOREWORD This document is one in a series of publications known as the INIS Reference Series. It is to be used in conjunction with the indexing manual 1) and the thesaurus 2) for the preparation of INIS input by national and regional centrea. The thesaurus and terminology charts in their first edition (Rev.0) were produced as the result of an agreement between the International Atomic Energy Agency (IAEA) and the European Atomic Energy Community (Euratom). Except for minor changesq the terminology and the interrela- tionships btween rms are those of the December 1969 edition of the Euratom Thesaurus 3) In all matters of subject indexing and ontrol, the IAEA followed the recommendations of Euratom for these charts. Credit and responsibility for the present version of these charts must go to Euratom. Suggestions for improvement from all interested parties. particularly those that are contributing to or utilizing the INIS magnetic-tape services are welcomed. These should be addressed to: The Thesaurus Speoialist/INIS Section Division of Scientific and Tohnioal Information International Atomic Energy Agency P.O. Box 590 A-1011 Vienna, Austria International Atomic Energy Agency Division of Sientific and Technical Information INIS Section June 1970 1) IAEA-INIS-12 (INIS: Manual for Indexing) 2) IAEA-INIS-13 (INIS: Thesaurus) 3) EURATOM Thesaurusq, Euratom Nuclear Documentation System. -

Eg9601814 Supergene Alteration of Magnetite And
EG9601814 SUPERGENE ALTERATION OF MAGNETITE AND PYRITE AND THE ROLE OF THEIR ALTERATION PRODUCTS IN THE FIXATION OF URANIUM FROM THE CIRCULATING MEDIA. BY MA EL GEMMIZI Nuclear Materials A uthority Cairo- Egypt. ABSTRACT. In most of the Egyptian altered radioactive granites, highly magnetic heavy particles were found to be radioactive. They are a mixture of several iron oxide minerals which are products of supergene alteration of the pre-existing hypogene iron-bearing minerals especially magnetite and pyrite. The end products of this supergene alteration are mainly hydrated iron oxide minerals limonite hematite and geothite. During the alteration, deformation and defects in the minerals structure took place , thereby promoting diffusion of the substitutional and interstitial ions (uranium) to words these sites The mechanism of the alteration of the hypogene iron-bearing minerals; magnetite and pyrite to form the secondary minerals hematite, limonite and geothite; the role of these minerals in fixing uranium from the ciculating media as well as the applicability of these minerals as indicators to the radioactivity of the host rocks were discussed. INTRODUCTION It is quite common that in all the altered rocks magnetite and to some extent pyrite which are present as accessories are suffering from some degrees of alteration. Magnetite and pyrite in the altered granite of Wadi Nugrus, El Missikat and £1 Aradyia, Eastern Desert was found by (1 & 2) to possess several degrees of alteration and crystal deformation. This means that both minerals are sensitive to postdepositional environmental changes. The granites of the present study were described as altered granites overlained by metasediments and underlained by magmatic bodies which invaded the granites itself by sills. -

United States Pmao" ICC Patented June 30, '1959 1 2 Adsorption on Manganese Dioxide, but It May on the 2,892,677 Other Hand He Left in the Solution
r. 2,892,677 United States Pmao" ICC Patented June 30, '1959 1 2 adsorption on manganese dioxide, but it may on the 2,892,677 other hand he left in the solution. If it is removed, a certain amount of manganese nitrate is formed from the ' SEPARATION OF URANIUM FROM THORIUM manganese dioxide in the solution and this nitrate reacts AND PROTACTINIUM with the sodium diethyldithiocarbamate to give manga William Kenneth Rodgerson Musgrave, Durham, Eng ' nese diethyldithiocarbamate, which must subsequently be land, assignor, by mesne assignments, to the United separated from the uranium. ,, . .States of America as represented by the United States Whether or not the protactinium has been removed, Atomic Energy Commission the solution is brought to a pH of between 2 and 3, for No Drawing. Application November 27, 1946 10 example by the addition of ammonia. The sodium di Serial No. 712,722 ethyldithiocarbamate is then dissolved in a solvent in which the subsequently formed uranyl diethyldithiocar '8 Claims. (Cl. 23—14.5) bamate is also soluble. Such a solvent is, for example, amyl acetate or methyl isobutyl ketone, the former being This invention relates to the separation of uranium 15 preferable for the reasons which will be indicated below. from thorium and protactinium. A mixture of these The sodium diethyldithiocarbamate is dissolved in amyl elements is obtained, for example, as the result of irradia acetate to form a solution containing 0.25% of the tion by neutrons of so-called thorium carbonate, which former, and this solution is then shaken up with the is a mixture of thorium oxide and thorium carbonate.