Meth/Amphetamine Use and Associated HIV: Implications for Global Policy and Public Health
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Mdma) in Non Conventional Matrices and Its Applications in Clinical Toxicology
Doctoral Thesis Doctorat Farmacología Departament de Farmacología, de Terapéutica i de Toxicologia DISTRIBUTION OF 3,4-METHYLENEDIOXYMETHAMPHETAMINE (MDMA) IN NON CONVENTIONAL MATRICES AND ITS APPLICATIONS IN CLINICAL TOXICOLOGY SIMONA PICHINI p Doctoral Thesis Doctorat Farmacología Departament de Farmacología, de Terapéutica i de Toxicologia DISTRIBUTION OF 3,4-METHYLENEDIOXYMETHAMPHETAMINE (MDMA) IN NON CONVENTIONAL MATRICES AND ITS APPLICATIONS IN CLINICAL TOXICOLOGY Doctoral thesis submitted by Simona Pichini as a partial fulfillment of the requirements for the degree of Doctor by the Universitat Autònoma de Barcelona. The studies included in this thesis have been realized under the direction of Dr. Rafael de la Torre Fornell and Dr. Magí Farré Albaladejo at the Pharmacology Unit of the Institut Municipal d'Investigació Mèdica (IMIM), Barcelona, Spain; and at the Drug Research and Control Department of the Istituto Superiore di Sanità, Roma, Italy. Doctorate Program of the Universitat Autònoma de Barcelona. Signature of Thesis Director Signature of Thesis Director (Dr. Magí Farré Albaladejo) (Dr. Rafael de la Torre Fornell) Signature of Doctorand (Simona Pichini) Yo soy yo y aquéllos a quienes amo Jorge Bucay To my friends, treasure of my life Acknowledgements Acknowledgements A number of people contributed to high extent to achieve the objectives of this Doctoral Thesis prepared between the two cities of Rome and Barcelona. This means that there are many people I’d the opportunity to meet, to work with, to share wonderful and terrible moments, and that I’d like to thank in these pages. First of all, to Dr. Piergiorgio Zuccaro, my “creator”, who always believed in me, supported my job and hardly fought to give me all the possible opportunities to grow up as an investigator; To Dr. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Evidence-Based Guidelines for the Pharmacological Management of Substance Abuse, Harmful Use, Addictio
444324 JOP0010.1177/0269881112444324Lingford-Hughes et al.Journal of Psychopharmacology 2012 BAP Guidelines BAP updated guidelines: evidence-based guidelines for the pharmacological management of substance abuse, Journal of Psychopharmacology 0(0) 1 –54 harmful use, addiction and comorbidity: © The Author(s) 2012 Reprints and permission: sagepub.co.uk/journalsPermissions.nav recommendations from BAP DOI: 10.1177/0269881112444324 jop.sagepub.com AR Lingford-Hughes1, S Welch2, L Peters3 and DJ Nutt 1 With expert reviewers (in alphabetical order): Ball D, Buntwal N, Chick J, Crome I, Daly C, Dar K, Day E, Duka T, Finch E, Law F, Marshall EJ, Munafo M, Myles J, Porter S, Raistrick D, Reed LJ, Reid A, Sell L, Sinclair J, Tyrer P, West R, Williams T, Winstock A Abstract The British Association for Psychopharmacology guidelines for the treatment of substance abuse, harmful use, addiction and comorbidity with psychiatric disorders primarily focus on their pharmacological management. They are based explicitly on the available evidence and presented as recommendations to aid clinical decision making for practitioners alongside a detailed review of the evidence. A consensus meeting, involving experts in the treatment of these disorders, reviewed key areas and considered the strength of the evidence and clinical implications. The guidelines were drawn up after feedback from participants. The guidelines primarily cover the pharmacological management of withdrawal, short- and long-term substitution, maintenance of abstinence and prevention of complications, where appropriate, for substance abuse or harmful use or addiction as well management in pregnancy, comorbidity with psychiatric disorders and in younger and older people. Keywords Substance misuse, addiction, guidelines, pharmacotherapy, comorbidity Introduction guidelines (e.g. -

Amphetamine-Type Stimulant Use Asia Region in the Southeast Blood-Borne Viruses Andof HIV Other and the Transmission Linkthe Between
25 The link between paper ANCD research amphetamine-type stimulant use and the transmission of HIV and other blood-borne viruses in the Southeast Asia region 25 The link between paper ANCD research amphetamine-type stimulant use and the transmission of HIV and other blood-borne viruses in the Southeast Asia region Andrea Fischer, Susan Curruthers, Robert Power, Steve Allsop and Louisa Degenhardt Macfarlane Burnet Institute for Medical Research and Public Health in partnership with the National Drug Research Institute, Curtin University A report prepared for the Australian National Council on Drugs, June 2012 © Australian National Council on Drugs 2013 This work is copyright. Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced by any process without the written permission of the publisher. Published by the Australian National Council on Drugs PO Box 205, Civic Square ACT 2608 Telephone: 02 6166 9600 Fax: 02 6162 2611 Email: [email protected] Website: www.ancd.org.au National Library of Australia Cataloguing-in-Publication data The link between amphetamine-type stimulant use and the transmission of HIV and other blood-borne viruses in the Southeast Asia region / Andrea Fischer … [et al.] ISBN: 9788770182799 ANCD research paper; 25. Includes bibliographic references. HIV (Viruses) — Southeast Asia — Transmission. HIV infections — Epidemiology — Southeast Asia. Intravenous drug abusers — Diseases — Southeast Asia. Intravenous drug abuse — Southeast Asia. Fischer, Andrea. Macfarlane Burnet Institute for Medical Research and Public Health (Melbourne, Vic.) National Drug Research Institute (Australia). Australian National Council on Drugs. 614.5993095 Editor: Julie Stokes Design: Starkis Design Acknowledgement: This work has been supported by funding from the Australian Government Department of Health and Ageing. -

Randomized Controlled Trial of Dexamphetamine Maintenance for the Treatment of Methamphetamine
RESEARCH REPORT doi:10.1111/j.1360-0443.2009.02717.x Randomized controlled trial of dexamphetamine maintenance for the treatment of methamphetamine dependenceadd_2717 146..154 Marie Longo1, Wendy Wickes1, Matthew Smout1, Sonia Harrison1, Sharon Cahill1 & Jason M. White1,2 Pharmacotherapies Research Unit, Drug and Alcohol Services South Australia, Norwood, South Australia, Australia1 and Discipline of Pharmacology, University of Adelaide, Adelaide, South Australia, Australia2 ABSTRACT Aim To investigate the safety and efficacy of once-daily supervised oral administration of sustained-release dexam- phetamine in people dependent on methamphetamine. Design Randomized, double-blind, placebo-controlled trial. Participants Forty-nine methamphetamine-dependent drug users from Drug and Alcohol Services South Australia (DASSA) clinics. Intervention Participants were assigned randomly to receive up to 110 mg/day sustained- release dexamphetamine (n = 23) or placebo (n = 26) for a maximum of 12 weeks, with gradual reduction of the study medication over an additional 4 weeks. Medication was taken daily under pharmacist supervision. Measurements Primary outcome measures included treatment retention, measures of methamphetamine consump- tion (self-report and hair analysis), degree of methamphetamine dependence and severity of methamphetamine withdrawal. Hair samples were analysed for methamphetamine using liquid chromatography-mass spectrometry. Findings Treatment retention was significantly different between groups, with those who received dexamphetamine remaining in treatment for an average of 86.3 days compared with 48.6 days for those receiving placebo (P = 0.014). There were significant reductions in self-reported methamphetamine use between baseline and follow-up within each group (P < 0.0001), with a trend to a greater reduction among the dexamphetamine group (P = 0.086). -

Management of Acute Withdrawal and Detoxification for Adults Who Misuse Methamphetamine: a Review of the Clinical Evidence and Guidelines
CADTH RAPID RESPONSE REPORT: SUMMARY WITH CRITICAL APPRAISAL Management of Acute Withdrawal and Detoxification for Adults who Misuse Methamphetamine: A Review of the Clinical Evidence and Guidelines Service Line: Rapid Response Service Version: 1.0 Publication Date: February 08, 2019 Report Length: 28 Pages Authors: Michelle Clark, Robin Featherstone Cite As: Management of Acute Withdrawal and Detoxification for Adults who Misuse Methamphetamine: A Review of the Clinical Evidence and Guidelines. Ottawa: CADTH; 2019 Feb. (CADTH rapid response report: summary with critical appraisal). ISSN: 1922-8147 (online) Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services. While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. -

Comparison Between Phenylephrine, Ephedrine and Mephentermine In
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 15, Issue 9 Ver. XIII (September. 2016), PP 52-58 www.iosrjournals.org Comparison Between Phenylephrine, Ephedrine And Mephentermine In Preventing Hypotension During Spinal Anesthesia For Caesarean Section And Their Effect On Fetal Outcome. Dr Sarika Sudhir Lonkar1, Dr Sonal Sagar Khatavkar2 Dr W S Thatte3 , Dr Naramaneni Santhi4 Department Of Anesthesia, Dr D Y Patil Medical College, Hospital And Research Centre Pimpri, Pune, Maharashtra, India1,2,3,4. Abstract Aim: To compare of the efficacy and safety of prophylactic bolus doses of intravenous Phenylephrine, Ephedrine, and Mephentermine for maintenance of arterial pressure during spinal anesthesia in caesarean section and their effects on neonate. Settings and Design: The present Prospective Randomized study was carried out in a tertiary care teaching hospital. A total of 90 pregnant women posted for elective caesarean section under spinal anesthesia were enrolled and randomly divided into three groups. Group P (phenylephrine) Group E (ephedrine) Group M (mephentermine) with 30 patients in each group. Materials and Methods: Group E received prophylactic bolus of ephedrine 6 mg IV, group M 6 mg IV mephentermine and group P 100 mcg IV of phenylephrine at the time of subarachnoid block. Hemodynamic variables like blood pressure and heart rate were recorded every 2 minutes up to delivery of baby and then every 5 minutes. Neonatal outcome was assessed using Apgar score at 1 and 5 minutes and neonatal umbilical cord blood pH values. Statistical Analysis Used: Comparitibility of groups are analyzed with analysis variance test to analyzed parametric data ‘P’ value < 0.05 was considered significant. -

(12) Patent Application Publication (10) Pub. No.: US 2015/0119330 A1 Mcgee Et Al
US 2015O1 19330A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0119330 A1 McGee et al. (43) Pub. Date: Apr. 30, 2015 (54) SUBSTRATES AND INHIBITORS OF PROLYL Publication Classification OLGOPEPTIDASE AND FIBROBLAST ACTIVATION PROTEIN AND METHODS OF (51) Int. Cl. USE C07K 7/06 (2006.01) (71) Applicant: The Board of Regents of the University C07K5/II (2006.01) of Oklahoma, Norman, OK (US) C07K5/09 (2006.01) (52) U.S. Cl. (72) Inventors: Patrick A. McGee, Oklahoma City, OK CPC ................. C07K 7/06 (2013.01); C07K5/0817 (US); Kenneth W. Jackson, Edmond, OK (US); Victoria J. Christiansen, (2013.01); C07K5/1019 (2013.01) Oklahoma City, OK (US) (21) Appl. No.: 14/398,886 (57) ABSTRACT (22) PCT Filed: May 3, 2013 Inhibitors of fibroblast activation protein alpha (FAP) and (86). PCT No.: PCT/US2013/0395.43 Prolyl Oligopeptidase (POP) are disclosed, along with their S371 (c)(1), use in various therapies related to conditions, diseases, and (2) Date: Nov. 4, 2014 disorders involving abnormal cell proliferation Such as malig nancies and angiogenesis, and in neural disorders such as Related U.S. Application Data Alzheimer's disease. Stalk portions of the inhibitor mol (60) Provisional application No. 61/643,001, filed on May ecules, and substrates of FAP and POP, are also disclosed and 4, 2012, provisional application No. 61/793,183, filed may be used, for example, in screening methods for identify on Mar. 15, 2013. ing Such inhibitors. US 201S/O119330 A1 Iaun3]+. ;0zzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzz… Patent Application Publication Apr. 30, 2015 Sheet 2 of 11 US 201S/O119330 A1 §§§§§§§§§§§ §§§§§§§§§§§§)§§§§§3 §§§§§§§§§§¶¡¿$¢$$$$$$§§§§§ $$$ §28, $$¢£€$£§§§§§§§§ §§§§§§§§§§§§§§§§§§ ? §§?ae, § § §§§§§§§§§§§§§§§§§§§§§??$$§§§§§§§§§§§§ $ §§§§§§§§§§§§§§§ a. -

Heterogeneity of Stimulant Dependence: a National Drug Abuse Treatment Clinical Trials Network Study
The American Journal on Addictions, 18: 206–218, 2009 Copyright C American Academy of Addiction Psychiatry ISSN: 1055-0496 print / 1521-0391 online DOI: 10.1080/10550490902787031 Heterogeneity of Stimulant Dependence: A National Drug Abuse Treatment Clinical Trials Network Study Li-Tzy Wu, ScD,1 Dan G. Blazer, MD, PhD,1 Ashwin A. Patkar, MD,1 Maxine L. Stitzer, PhD,2 Paul G. Wakim, PhD,3 Robert K. Brooner, PhD2 1Duke University School of Medicine, Department of Psychiatry and Behavioral Sciences, Duke Clinical Research Institute, Durham, North Carolina 2Johns Hopkins University School of Medicine, Department of Psychiatry and Behavioral Sciences, Baltimore, Maryland 3National Institute on Drug Abuse, Bethesda, Maryland We investigated the presence of DSM-IV subtyping for of disorders has noteworthy advantages because it facilitates dependence on cocaine and amphetamines (with versus with- research, improves communications among clinicians and out physical dependence) among outpatient stimulant users researchers, and serves as a necessary tool for collecting enrolled in a multisite study of the Clinical Trials Network 1 (CTN). Three mutually exclusive groups were identified: and communicating public health statistics. The categorical primary cocaine users (n = 287), primary amphetamine users classification, however, also constitutes a primary limitation (n = 99), and dual users (cocaine and amphetamines; n = because it works best when all members of a given diagnosis 29). Distinct subtypes were examined with latent class and are homogeneous and when there are clear boundaries between logistic regression procedures. Cocaine users were distinct distinct diagnoses.1 from amphetamine users in age and race/ethnicity. There were four distinct classes of primary cocaine users: non- DSM-IV acknowledges this limitation and further suggests dependence (15%), compulsive use (14%), tolerance and the presence of heterogeneity among individuals who share compulsive use (15%), and physiological dependence (toler- a diagnosis. -

Director's Report 2/00
Director's Report 2/00 National Institute on Drug Abuse Director's Report to the National Advisory Council on Drug Abuse February, 2000 Index Research Findings Basic Research Behavioral Research Treatment Research and Development Research on AIDS and Other Medical Consequences of Drug Abuse Epidemiology, Etiology and Prevention Research Services Research Intramural Research Program Activities Review Activities Congressional Affairs International Activities Meetings and Conferences Media and Education Activities Planned Meetings Publications Staff Highlights Grantee Honors [Office of Director] [First Report Section] https://archives.drugabuse.gov/DirReports/DirRep200/DirectorRepIndex.html[11/17/16, 9:37:32 PM] Director's Report 2/00 Archive Home | Accessibility | Privacy | FOIA (NIH) | Current NIDA Home Page The National Institute on Drug Abuse (NIDA) is part of the National Institutes of Health (NIH) , a component of the U.S. Department of Health and Human Services. Questions? _ See our Contact Information. https://archives.drugabuse.gov/DirReports/DirRep200/DirectorRepIndex.html[11/17/16, 9:37:32 PM] Director's Report 2/00 - Basic Research National Institute on Drug Abuse Director's Report to the National Advisory Council on Drug Abuse February, 2000 Research Findings Basic Research A Brain Protein Is Involved in Switching On Cocaine Addiction Chronic exposure to cocaine causes the delta-FosB transcription factor to be expressed persistently in the nucleus accumbens. Thus, researchers hypothesized that delta-FosB may mediate some of the long-lived increases in sensitivity to the stimulant and rewarding effects of cocaine. Dr. Eric Nestler of Yale University and his colleagues tested whether delta-FosB expression actually increased the responsiveness to cocaine by generating genetically altered mice that produced large quantities of delta-FosB in the nucleus accumbens. -
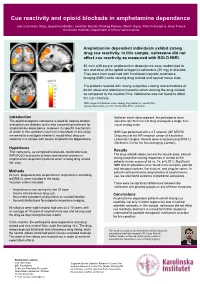
Cue Reactivity and Opioid Blockade in Amphetamine Dependence
Cue reactivity and opioid blockade in amphetamine dependence Joar Guterstam, Nitya Jayaram-Lindström, Jonathan Berrebi, Predrag Petrovic, Martin Ingvar, Peter Fransson & Johan Franck Karolinska Institutet, Department of clinical neuroscience Amphetamine dependent individuals exhibit strong drug cue reactivity. In this sample, naltrexone did not affect cue reactivity as measured with BOLD fMRI. 40 men with severe amphetamine dependence were randomized to one oral dose of the opioid antagonist naltrexone (50 mg) or placebo. They were then examined with functional magnetic resonance imaging (fMRI) while viewing drug related and neutral movie clips. The patients reacted with strong subjective craving and activations of limbic areas and attentional networks when viewing the drug related, as compared to the neutral, films. Naltrexone was not found to affect the cue reactivity. fMRI image of activations when viewing drug related vs. neutral films (whole-brain results, p<0.05, Family-Wise Error corrected). Introduction Between each video segment, the participants were The opioid antagonist naltrexone is used for treating alcohol asked to rate their level of drug craving on a single item and opioid use disorder, but is also a promising treatment for visual analog scale. amphetamine dependence. However, its specific mechanism of action in this condition is not well understood. In this study, fMRI was performed with a 3 T scanner (GE MR750 we aimed to investigate whether it would affect drug cue Discovery) at the MR research center of Karolinska reactivity in a sample with severe amphetamine dependence. University Hospital. Results were analyzed using SPM 12 (Wellcome Center for Neuroimaging, London). Hypothesis That naltrexone, as compared to placebo, would attenuate Results fMRI BOLD activations of brain motivational systems in The drug-related videos, but not the neutral ones, elicited amphetamine dependent patients when viewing drug related strong subjective craving responses in almost all the film clips. -

Substance Abuse and Rehabilitation Dovepress Open Access to Scientific and Medical Research
Substance Abuse and Rehabilitation Dovepress open access to scientific and medical research Open Access Full Text Article CommENtarY Substance abuse and rehabilitation: responding to the global burden of diseases attributable to substance abuse Li-Tzy Wu* Abstract: Alcohol, tobacco, and illegal drug use are pervasive throughout the world. Substance Department of Psychiatry and use problems are among the major contributors to the global disease burden, which includes Behavioral Sciences, Duke University disability and mortality. The benefits of treatment far outweigh the economic costs. Despite School of Medicine, Duke University the availability of treatment services, however, the vast majority of people with substance use Medical Center, Durham, NC, USA disorders do not seek or use treatment. Barriers to and unmet need for evidence-based treat- * Li-Tzy Wu is the Editor-in-Chief ment are widespread even in the United States. Women, adolescents, and young adults are of Substance Abuse and Rehabilitation especially vulnerable to adverse effects from substance abuse, but they face additional barriers to getting evidence-based treatment or other social/medical services. Substance use behaviors and the diseases attributable to substance use problems are preventable and modifiable. Yet the ever-changing patterns of substance use and associated problems require combined research and policy-making efforts from all parts of the world to establish a viable knowledge base to inform for prevention, risk-reduction intervention, effective use of evidence-based treatment, and rehabilitation for long-term recovery. The new international, open-access, peer-reviewed Substance Abuse and Rehabilitation (SAR) journal strives to provide an effective platform for sharing ideas for solutions and disseminating research findings globally.