Hybrid Hazelnut Oil Characteristics and Its Potential Oleochemical Application1 Y.X
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hazelnut: a Valuable Resource
International Journal of Food Engineering Vol. 6, No. 2, December 2020 Hazelnut: A Valuable Resource Raquel P. F. Guiné and Paula M. Reis Correia CI&DETS/ESAV, Polytechnic Institute of Viseu, Department of Food Industry, Viseu, Portugal Email: [email protected], [email protected] Abstract—Hazel (Corylus avellana L.) nutshell is one of the remaining 90% is used for industrial purposes as shelled most consumed and most appreciated nut fruit all over the nuts [7], [8]. world. It is believed to have constituted a basic food in early Similarly, to other nuts, hazelnuts are included in the prehistory, in temperate zones of the globe, such as for dietary guidelines of several countries, due to their high example Europe. Presently the hazelnut production is nutritional content and bioavailability of those nutrients. mainly concentrated on the Black Sea coast of Turkey, but other countries are also important producers, like for Furthermore, the bioactive molecules present in hazelnuts example Portugal, situated on the western Europe, in the have been reported as having several benefits for the Iberian Peninsula. The objective of this work is to make a human health [9]–[11]. review about the worldwide importance of hazelnut, their usages, including gastronomic and industrial applications, II. CONSUMPTION & MAIN USAGES as well as some ways that allow adding value to this fruit, making it an even more valuable resource. The advantages From the Neolithic era, in the regions of Europe and include higher income for produces, lower environmental the Caucasus, hazelnut has been used for human impacts and valorisation of residues improving consumption. -

Essential Wholesale & Labs Carrier Oils Chart
Essential Wholesale & Labs Carrier Oils Chart This chart is based off of the virgin, unrefined versions of each carrier where applicable, depending on our website catalog. The information provided may vary depending on the carrier's source and processing and is meant for educational purposes only. Viscosity Absorbtion Comparible Subsitutions Carrier Oil/Butter Color (at room Odor Details/Attributes Rate (Based on Viscosity & Absorbotion Rate) temperature) Description: Stable vegetable butter with a neutral odor. High content of monounsaturated oleic acid and relatively high content of natural antioxidants. Offers good oxidative stability, excellent Almond Butter White to pale yellow Soft Solid Fat Neutral Odor Average cold weather stability, contains occlusive properties, and can act as a moistening agent. Aloe Butter, Illipe Butter Fatty Acid Compositon: Palmitic, Stearic, Oleic, and Linoleic Description: Made from Aloe Vera and Coconut Oil. Can be used as an emollient and contains antioxidant properties. It's high fluidiy gives it good spreadability, and it can quickly hydrate while Aloe Butter White Soft Semi-Solid Fat Neutral Odor Average being both cooling and soothing. Fatty Acid Almond Butter, Illipe Butter Compostion: Linoleic, Oleic, Palmitic, Stearic Description: Made from by combinging Aloe Vera Powder with quality soybean oil to create a Apricot Kernel Oil, Broccoli Seed Oil, Camellia Seed Oil, Evening Aloe Vera Oil Clear, off-white to yellow Free Flowing Liquid Oil Mild musky odor Fast soothing and nourishing carrier oil. Fatty Acid Primrose Oil, Grapeseed Oil, Meadowfoam Seed Oil, Safflower Compostion: Linoleic, Oleic, Palmitic, Stearic Oil, Strawberry Seed Oil Description: This oil is similar in weight to human sebum, making it extremely nouirshing to the skin. -
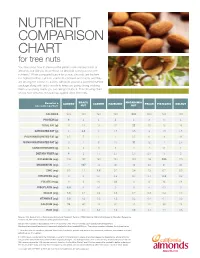
Nutrient Comparison Chart
NUTRIENT COMPARISON CHART for tree nuts You may know how to measure the perfect one-ounce portion of almonds, but did you know those 23 almonds come packed with nutrients? When compared ounce for ounce, almonds are the tree nut highest in fiber, calcium, vitamin E, riboflavin and niacin, and they are among the lowest in calories. Almonds provide a powerful nutrient package along with tasty crunch to keep you going strong, making them a satisfying snack you can feel good about. The following chart shows how almonds measure up against other tree nuts. BRAZIL MACADAMIA Based on a ALMOND CASHEW HAZELNUT PECAN PISTACHIO WALNUT one-ounce portion1 NUT NUT CALORIES 1602 190 160 180 200 200 160 190 PROTEIN (g) 6 4 4 4 2 3 6 4 TOTAL FAT (g) 14 19 13 17 22 20 13 19 SATURATED FAT (g) 1 4.5 3 1.5 3.5 2 1.5 1.5 POLYUNSATURATED FAT (g) 3.5 7 2 2 0.5 6 4 13 MONOUNSATURATED FAT (g) 9 7 8 13 17 12 7 2.5 CARBOHYDRATES (g) 6 3 9 5 4 4 8 4 DIETARY FIBER (g) 4 2 1.5 2.5 2.5 2.5 3 2 POTASSIUM (mg) 208 187 160 193 103 116 285 125 MAGNESIUM (mg) 77 107 74 46 33 34 31 45 ZINC (mg) 0.9 1.2 1.6 0.7 0.4 1.3 0.7 0.9 VITAMIN B6 (mg) 0 0 0.1 0.2 0.1 0.1 0.3 0.2 FOLATE (mcg) 12 6 20 32 3 6 14 28 RIBOFLAVIN (mg) 0.3 0 0.1 0 0 0 0.1 0 NIACIN (mg) 1.0 0.1 0.4 0.5 0.7 0.3 0.4 0.3 VITAMIN E (mg) 7.3 1.6 0.3 4.3 0.2 0.4 0.7 0.2 CALCIUM (mg) 76 45 13 32 20 20 30 28 IRON (mg) 1.1 0.7 1.7 1.3 0.8 0.7 1.1 0.8 Source: U.S. -

Information About Oils the National Edible Oil Distributors’ Association
Information About Oils The National Edible Oil Distributors’ Association Sunflower Oil Sunflower Oil - Information About Oils Introduction Nutritional Profile Whilst the vibrant strong sunflower is recognised Due to its nutritional profile, sunflower oil can bear worldwide for its beauty, it is also an important the following nutrition claims: source of food. Sunflower oil is a valued and Nutrition Claims High polyunsaturated fat (more healthy vegetable oil and sunflower seeds are en- than 45% of the fatty acids are from polyunsaturat- joyed as a healthy, tasty snack and nutritious ingre- ed fats, which represent more than 20% of the en- dient to many foods. ergy content) High unsaturated fat (more than 70% Sunflower oil is obtained from the seeds within the of the fatty acids are from unsaturated fats, which brown hub in the centre of the sunflower plant. represent more than 20% of the energy content) Each flower can develop up to 2000 sunflower High vitamin E (more than 30% of Recommended seeds. The oil is pale yellow in colour but contains Daily Allowances (RDA) of vitamin E set at 12 mg/day a level of natural waxes that give the oil a ‘cloudy’ Health claims – Positive EFSA opinion Linoleic acid appearance at cooler temperatures. These waxes (omega-6 fatty acids) contributes to the mainte- can be removed by a process commonly known as nance of normal blood cholesterol concentrations. winterisation. Essential fatty acids (omega-3 and omega-6 fatty The wild sunflower is native to North America but acids) are needed for the normal growth of children. commercialisation of the plant took place in Rus- Vitamin E protects lipids, proteins and DNA against sia. -

List of Legumes
Healthy Oils & SmokePoints When it comes to the cooking oil in your cupboard, there is a huge difference in healthfulness depending on how the oil is stored and how it will be used. First let’s get some definitions for commonly used terms on labels and discussions about oils. Term Definition Cold Pressed Extracted without using heat. Extracted using a screw-type machine that presses the oil out. Can be done Expeller Pressed slowly, with very little heat, or can be done quickly with lots of friction and high temperatures. The first cold pressing which contains the best tasting and most healthful oils. Must contain less than 1% acids. By definition, this is cold-pressed and first Extra Virgin pressed, so don’t need to see these terms on the label. Must say 100% extra virgin, or may be a blend. The first cold pressing, but can contain a little more acids than the extra-virgin Virgin (1-3 percent). Seeds that have been genetically manipulated to decrease the amount of High Oleic essential fatty acids so that they have a longer shelf life. Are left in their state after pressing – no filtering. These oils tend to be more Unrefined Oils flavorful and richer in nutrients, however they have a very low smoke point. Oils have their impurities filtered out, to increase stability and allow for higher Refined temperature cooking. The processing can use toxic solvents, caustic soda, bleaches and phosphoric acid. Smoke Point Stage at which a heated oil begins to smoke, just before it bursts into flames. HEALTHY OILS & SMOKE POINTS PAGE | 1 © 2021 Health-Naturally.org Term Definition Oil should smell and taste like the food it came from. -

Filbert European Corylus Avellana Corylus Avellana Commonly Called
Filbert European Corylus avellana Corylus avellana commonly called European Filbert, European hazel, cobnut and Harry Lauder’s walking stick is a deciduous, thicket-forming, multi-trunked suckering shrub. Common names of filbert and hazel are likely interchangeable. Hazel is more often used in reference to wild specimens and filbert is more likely to be used in reference to cultivated plants. The filbert nuts to be produced in commerce primarily come from plants (C. avellano x C. maxima). ‘Contorta’, commonly called contorted filbert, corkscrew hazel or Harry Lauder’s Walking Stick, is contorted version of the species plant. It was discovered growing as a sport in an English hedgerow In the mid-1800s by Victorian Gardner Cannon Ellacombe. This plant was given the common name of Harry Lauder’s walking stick in the 1900s in honor of the Scottish entertainer Harry Lauder. The European Filbert leaves are dark green, slightly covered with fine soft hairs above and beneath; alternate; 2-4” in length, somewhat circular to egg – shaped or heart – shaped, abruptly tapers to a point at apex, edge doubly toothed, often with lobes, petiole ¼” to ½” long. The twigs are brown, glandular – hairy. Buds green to brown, hairless with hairy scale; overlapping, egg shaped to round. Flowers/Fruit: Flowers monoecious; male flowers are large (2”to 3”) catkins, yellow – brown, late winter to early spring blooming; female flowers inconspicuous. Fruit a nut; nuts inside involucre, which is toothed or lubed and nearly the length of the nut; ¾” in length; edible fruit grown commercially as a crop. European Filbert bark is pale to gray – brown, smoother with age, not an ornamental feature. -

Nutritive Value and Degradability of Leaves from Temperate Woody Resources for Feeding Ruminants in Summer
3rd European Agroforestry Conference Montpellier, 23-25 May 2016 Silvopastoralism (poster) NUTRITIVE VALUE AND DEGRADABILITY OF LEAVES FROM TEMPERATE WOODY RESOURCES FOR FEEDING RUMINANTS IN SUMMER Emile JC 1*, Delagarde R 2, Barre P 3, Novak S 1 Corresponding author: [email protected] mailto:(1) INRA, UE 1373 FERLUS, 86600 Lusignan, France (2) INRA, UMR 1348 INRA-Agrocampus Ouest, 35590 Saint-Gilles, France (3) INRA, UR 4 URP3F, 86600 Lusignan, France 1/ Introduction Integrating agroforestry in livestock farming systems may be a real opportunity in the current climatic, social and economic conditions. Trees can contribute to improve welfare of grazing ruminants. The production of leaves from woody plants may also constitute a forage resource for livestock (Papanastasis et al. 2008) during periods of low grasslands production (summer and autumn). To know the potential of leaves from woody plants to be fed by ruminants, including dairy females, the nutritive value of these new forages has to be evaluated. References on nutritive values that already exist for woody plants come mainly from tropical or Mediterranean climatic conditions (http://www.feedipedia.org/) and very few data are currently available for the temperate regions. In the frame of a long term mixed crop-dairy system experiment integrating agroforestry (Novak et al. 2016), a large evaluation of leaves from woody resources has been initiated. The objective of this evaluation is to characterise leaves of woody forage resources potentially available for ruminants (hedgerows, coppices, shrubs, pollarded trees), either directly by browsing or fed after cutting. This paper presents the evaluation of a first set of 12 woody resources for which the feeding value is evaluated through their protein and fibre concentrations, in vitro digestibility (enzymatic method) and effective ruminal degradability. -

Descriptors for Hazelnut (Corylus Avellana L.)
Descriptors for Hazelnut(Corylus avellana L.) List of Descriptors Allium (E, S) 2001 Pearl millet (E/F) 1993 Almond (revised)* (E) 1985 Pepino (E) 2004 Apple* (E) 1982 Phaseolus acutifolius (E) 1985 Apricot* (E) 1984 Phaseolus coccineus* (E) 1983 Avocado (E/S) 1995 Phaseolus lunatus (P) 2001 Bambara groundnut (E, F) 2000 Phaseolus vulgaris* (E, P) 1982 Banana (E, S, F) 1996 Pigeonpea (E) 1993 Barley (E) 1994 Pineapple (E) 1991 Beta (E) 1991 Pistachio (A, R, E, F) 1997 Black pepper (E/S) 1995 Pistacia (excluding Pistacia vera) (E) 1998 Brassica and Raphanus (E) 1990 Plum* (E) 1985 Brassica campestris L. (E) 1987 Potato variety* (E) 1985 Buckwheat (E) 1994 Quinua* (E) 1981 Cañahua (S) 2005 Rambutan 2003 Capsicum (E/S) 1995 Rice* (E) 2007 Cardamom (E) 1994 Rocket (E, I) 1999 Carrot (E, S, F) 1998 Rye and Triticale* (E) 1985 Cashew* (E) 1986 Safflower* (E) 1983 Cherry* (E) 1985 Sesame (E) 2004 Chickpea (E) 1993 Setaria italica and S. pumilia (E) 1985 Citrus (E, F, S) 1999 Shea tree (E) 2006 Coconut (E) 1995 Sorghum (E/F) 1993 Coffee (E, S, F) 1996 Soyabean* (E/C) 1984 Cotton (revised)* (E) 1985 Strawberry (E) 1986 Cowpea (E, P)* 1983 Sunflower* (E) 1985 Cultivated potato* (E) 1977 Sweet potato (E/S/F) 1991 Date Palm (F) 2005 Taro (E, F, S) 1999 Durian (E) 2007 Tea (E, S, F) 1997 Echinochloa millet* (E) 1983 Tomato (E, S, F) 1996 Eggplant (E/F) 1990 Tropical fruit (revised)* (E) 1980 Faba bean* (E) 1985 Ulluco (S) 2003 Fig (E) 2003 Vigna aconitifolia and V. -

Effects of Processing Treatments on Nutritional Quality of Raw Almond (Terminalia Catappa Linn.) Kernels
Available online a t www.pelagiaresearchlibrary.com Pelagia Research Library Advances in Applied Science Research, 2016, 7(1):1-7 ISSN: 0976-8610 CODEN (USA): AASRFC Effects of processing treatments on nutritional quality of raw almond (Terminalia catappa Linn.) kernels *Makinde Folasade M. and Oladunni Subomi S. Department of Food Science and Technology, Bowen University, Iwo, Osun State, Nigeria _____________________________________________________________________________________________ ABSTRACT Almond (Terminalia catappa Linn) is one of the lesser utilized oil kernel distributed throughout the tropics including Nigeria ecosystem. In this research work, the effects of soaking, blanching, autoclaving and roasting on the proximate, mineral, vitamin and anti-nutritional concentrations of almond kernel were determined. The result of chemical composition revealed that raw almond kernel contained 11.93% moisture, 23.0% crude protein, 48.1% crude fat, 2.43% crude fiber, 2.69% ash, 12.0% carbohydrate, 0.35mg/100g thiamine, 0.15mg/100g riboflavin, 0.19mg/100g niacin and minerals among which the most important are potassium (9.87 mg/100g), calcium (4.66 mg/100g) and magnesium (4.45 mg/100g). Tannin, phytate and oxalate concentration in raw almond kernel were 0.15, 0.13 and 0.15mg/100g respectively. Increase in ash and fiber was noted for treated samples with time compared to raw almond. Compared to untreated kernels, soaking, blanching and autoclaving decreased fat content but there was increase during roasting of the kernels. Mineral concentrations were significantly increased by various treatments compared to raw kernel. However, roasting for 15 min resulted in highest increase in potassium (41.2 percent), calcium (45.1 percent), phosphorus (43.3 percent) and magnesium (43.6 percent). -

Medicinal Use of Sunflower Oil and Present Status of Sunflower in Pakistan: a Review Study
Sci., Tech. and Dev., 31 (2): 99-106, 2012 Medicinal Use of Sunflower Oil and Present Status of Sunflower in Pakistan: A Review Study MUHAMMAD ARSHAD AND MUHAMMAD AMJAD Oilseeds Research Program, National Agricultural Research Centre, Islamabad, Pakistan. Abstract Sunflower {Helianthus annus (L.)} contributes 30% in domestic edible oil crop and has become the most important oil crop. A case study was planned to examine and review the current status of its production in Pakistan and sunflower oil use in medicine and nutrition. This study deals with the medicinal values of its oil with regards to its benefits and side effects. Sunflower seeds contain 20-30% protein as well as iron, B vitamins, vitamin A, calcium, nitrogen and phosphorus. The constituents of the seeds are a volatile oil, carbonate of potash, tannin and excellent sources of the B vitamins (B1, B3 and B6) including niacin and pantothenate. Sunflower oil is high in the essential vitamin E and low in saturated fat. Two common types of sunflower oil are linoleic and high oleic. Linoleic oil has high levels of polyunsaturated fat. It is also known for having a clean taste and low levels of trans fat. High oleic sunflower oils are classified as having monounsaturated levels of 80% and above. The iron-rich sunflower seeds are, by weight, 47% fat and 20-30 % protein. The seeds have more than 48 calories/tablespoon. Sunflower seeds have more iron than any other food except for liver and egg yolk. Sunflower seeds are more commonly eaten as a healthy snack than as part of a meal. -

Hazelnuts Resistant to Eastern Filbert Blight: Are We There Yet?
Hazelnuts Resistant to Eastern Filbert Blight: Are We There Yet? Thomas J. Molnar, Ph.D. Plant Biology and Pathology Dept. Rutgers University The American Chestnut Society Annual Meeting October 22, 2011 Nut Tree Breeding at Rutgers Based on the tremendous genetic improvements demonstrated in several previously underutilized turf species, Dr. C. Reed Funk strongly believed similar work could be done with nut trees Title of project started in 1996: Underutilized Perennial Food Crops Genetic Improvement Tom Molnar and Reed Funk Program Adelphia Research Farm August 2001 Nut Breeding at Rutgers Starting in 1996, species of interest included – black walnuts, Persian walnuts and heartnuts – pecans, hickories Pecan shade trial – chestnuts, Adelphia 2000 – almonds, – hazelnuts We built a germplasm collection of over 25,000 trees planted across five Rutgers research farms – Cream Ridge Fruit Research Farm (Cream Ridge, NJ) – Adelphia Research Farm (Freehold, NJ) Pecan shade trial Adelphia 2008 – HF1, HF2, HF3 (North Brunswick, NJ) Nut Breeding at Rutgers Goals – Identify species that show the greatest potential for New Jersey and Mid- Atlantic region – Develop breeding program to create superior well- adapted cultivars that reliably produce high- quality, high-value crops • while requiring reduced inputs of pesticides, fungicides, management, etc. Nut Breeding at Rutgers While most species showed great promise for substantial improvement, we had to narrow our focus to be most effective Hazelnuts stood out as the species where we could make significant contributions in a relatively short period of time – Major focus since 2000 Hazelnuts at Rutgers Why we chose to focus on hazelnuts: – success of initial plantings made in 1996/1997 with few pests and diseases – short generation time and small plant size (4 years from seed to seed) – wide genetic diversity and the ability to hybridize different species – ease of making controlled crosses – backlog of information and breeding advances – existing technologies and markets for nuts Hazelnuts: Corylus spp. -

This Article Appeared in a Journal Published by Elsevier. the Attached Copy Is Furnished to the Author for Internal Non-Commerci
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/authorsrights Author's personal copy Scientia Horticulturae 171 (2014) 91–94 Contents lists available at ScienceDirect Scientia Horticulturae journal homepage: www.elsevier.com/locate/scihorti Short communication Effect of coconut water and growth regulator supplements on in vitro propagation of Corylus avellana L ∗ M.A. Sandoval Prando, P. Chiavazza, A. Faggio, C. Contessa Dipartimento di Scienze Agrarie Forestali e Alimentari (DISAFA), Università degli Studi di Torino, Largo Paolo Braccini, 2-10095 Grugliasco, TO, Italy a r t i c l e i n f o a b s t r a c t Article history: Corylus avellana L. represents an economically important crop in the European Union. Several protocols of Received 3 February 2014 hazelnut micropropagation have been tested but have not yet provided effective methods to allow large- Received in revised form 27 March 2014 scale propagation for commercial purposes, due to poor proliferation and yield. The aim of this work Accepted 31 March 2014 was to study the in vitro effects of coconut water (CW) in combinations with gibberellins and cytokinins Available online 19 April 2014 on proliferation and growth of hazelnut in vitro and the effect of explants orientation on shoot growth.