International Randomised Controlled Trial Evaluating Metabolic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ASH NEW ZEALAND Tobacco Returns Analysis 2013 1 TOBACCO
ASH NEW ZEALAND Tobacco Returns Analysis 2013 TOBACCO RETURNS ANALYSIS 2013 Tobacco manufacturers and importers in New Zealand are required to file their annual tobacco returns pursuant to section 35 of the Smokefree Environments Act 1990. The tobacco returns consist of two components – Schedule A and Schedule B. Schedule A is the information about ingredients and amounts used in their tobacco products. Schedule B is the company list of all their products, retail price and sale quantity We analysed data from Schedule B in order to understand the brand positioning of the major tobacco companies. This includes analysis of retail price, sale volume and estimated sales returned of all tobacco brands. Market share also is estimated based on this data. This analysis accounted for cigarette and loose leaf tobacco products sold within New Zealand (or domestic market). Information provided by tobacco companies may vary and hence the results of the analysis. British American Tobacco New Zealand (BATNZ) provided recommended retail prices as at January 2013 while Imperial Tobacco New Zealand and Philip Morris provided theirs as at December 2013. This had resulted in artificially higher estimates of average retail price and sale return for BATNZ products due to the January 2014 tax increase. Having considered this, most of the results on overall tobacco market in this analysis were presented according to the sale quantity and hence better accuracy. Fourteen companies filed their tobacco returns in 2013. As this analysis only included cigarette and loose leaf tobacco products, three companies were excluded as they sold cigar or other tobacco products. Tobacco Returns 2012 can be accessed through the National Drug Policy New Zealand website (http://www.ndp.govt.nz/moh.nsf/indexcm/ndp-tobacco-returns-2012 ). -

North Dakota Office of State Tax Commissioner Tobacco Directory List of Participating Manufacturers (Listing by Brand) As of May 24, 2019
North Dakota Office of State Tax Commissioner Tobacco Directory List of Participating Manufacturers (Listing by Brand) As of May 24, 2019 **RYO: Roll-Your-Own Brand Name Manufacturer 1839 U.S. Flue-Cured Tobacco Growers, Inc. 1839 RYO U.S. Flue-Cured Tobacco Growers, Inc. 1st Class U.S. Flue-Cured Tobacco Growers, Inc. American Bison RYO Wind River Tobacco Company, LLC Amsterdam Shag RYO Peter Stokkebye Tobaksfabrik A/S Ashford RYO Von Eicken Group Bali Shag RYO Commonwealth Brands, Inc. Baron American Blend Farmer’s Tobacco Co of Cynthiana, Inc. Basic Philip Morris USA, Inc. Benson & Hedges Philip Morris USA, Inc. Black & Gold Sherman’s 1400 Broadway NYC, LLC Bo Browning RYO Top Tobacco, LP Bugler RYO Scandinavian Tobacco Group Lane Limited Bull Brand RYO Von Eicken Group Cambridge Philip Morris USA, Inc. Camel R.J. Reynolds Tobacco Company Camel Wides R.J. Reynolds Tobacco Company Canoe RYO Wind River Tobacco Company, LLC Capri R.J. Reynolds Tobacco Company Carlton R.J. Reynolds Tobacco Company CF Straight Virginia RYO Von Eicken Group Charles Fairmon RYO Von Eicken Group Chesterfield Philip Morris USA, Inc. Chunghwa Konci G & D Management Group (USA) Inc. Cigarettellos Sherman’s 1400 Broadway NYC, LLC Classic Sherman’s 1400 Broadway NYC, LLC Classic Canadian RYO Top Tobacco, LP Commander Philip Morris USA, Inc. Crowns Commonwealth Brands Inc. Custom Blends RYO Wind River Tobacco Company, LLC Brand Name Manufacturer Danish Export RYO Peter Stokkebye Tobaksfabrik A/S Dark Fired Shag RYO Von Eicken Group Dave’s Philip Morris USA, Inc. Davidoff Commonwealth Brands, Inc. Djarum P.T. -

Cigarettes and Tobacco Products Removed from the California Tobacco Directory by Brand
Cigarettes and Tobacco Products Removed From The California Tobacco Directory by Brand Brand Manufacturer Date Comments Removed #117 - RYO National Tobacco Company 10/21/2011 5/6/05 Man. Change from RBJ to National Tobacco Company 10/20's (ten-twenty's) M/s Dhanraj International 2/6/2012 2/2/05 Man. Name change from Dhanraj Imports, Inc. 10/20's (ten-twenty's) - RYO M/s Dhanraj International 2/6/2012 1st Choice R.J. Reynolds Tobacco Company 5/3/2010 Removed 5/2/08; Reinstated 7/11/08 32 Degrees General Tobacco 2/28/2010 4 Aces - RYO Top Tobacco, LP 11/12/2010 A Touch of Clove Sherman 1400 Broadway N.Y.C. Inc. 9/25/2009 AB Rimboche' - RYO Daughters & Ryan, Inc. 6/18/2010 Ace King Maker Marketing 5/21/2020 All American Value Philip Morris, USA 5/5/2006 All Star Liberty Brands, LLC 5/5/2006 Alpine Philip Morris, USA 8/14/2013 Removed 5/4/07; Reinstated 5/8/09 Always Save Liberty Brands, LLC 5/4/2007 American R.J. Reynolds Tobacco Company 5/6/2005 American Bison Wind River Tobacco Company, LLC 9/22/2015 American Blend Mac Baren Tobacco Company 5/4/2007 American Harvest Sandia Tobacco Manufacturers, Inc. 8/31/2016 American Harvest - RYO Truth & Liberty Manufacturing 8/2/2016 American Liberty Les Tabacs Spokan 5/12/2006 Amphora - RYO Top Tobacco, LP 11/18/2011 Andron's Passion VCT 5/4/2007 Andron's Passion VCT 5/4/2007 Arango Sportsman - RYO Daughters & Ryan, Inc. 6/18/2010 Arbo - RYO VCT 5/4/2007 Ashford Von Eicken Group 5/8/2009 Ashford - RYO Von Eicken Group 12/23/2011 Athey (Old Timer's) Daughters & Ryan, Inc. -

Tobacco Dependency Needs Assessment
Tobacco Dependence Needs Assessment January 2019 Produced by Claire Mulrenan, Public Health Registrar, Kent County Council Andrew Scott-Clark, Director of Public Health, Kent County Council Contents Executive Summary Background and Introduction ............................................................................................ 3 Epidemiology .................................................................................................................... 3 Smoking Cessation Services ............................................................................................... 4 1 Background ................................................................................ 6 2 Introduction ............................................................................... 6 Summary – Part 1 ................................................................................................................ 17 3 Epidemiology ............................................................................ 18 Summary – Part 2 ................................................................................................................ 31 4 Smoking Cessation Services ...................................................... 32 Summary and Recommendations – Part 3 ........................................................................... 41 Appendix 1 – Global Burden of disease ................................................................................ 43 Appendix 2- Cigarettes vs e-cigarettes ................................................................................ -

Smoking Cessation Interventions in Pregnancy and Postpartum Care
Evidence Report/Technology Assessment Number 214 Smoking Cessation Interventions in Pregnancy and Postpartum Care Evidence-Based Practice Evidence Report/Technology Assessment Number 214 Smoking Cessation Interventions in Pregnancy and Postpartum Care Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 540 Gaither Road Rockville, MD 20850 www.ahrq.gov Contract No. 290-2007-10065-I Prepared by: Vanderbilt Evidence-based Practice Center Nashville, TN Investigators: Frances E. Likis, Dr.P.H., N.P., CNM Jeffrey C. Andrews, M.D. Christopher J. Fonnesbeck, Ph.D. Katherine E. Hartmann, M.D., Ph.D. Rebecca N. Jerome, M.L.I.S., M.P.H. Shannon A. Potter, M.L.I.S. Tanya S. Surawicz, M.P.H. Melissa L. McPheeters, Ph.D., M.P.H. AHRQ Publication No. 14-E001-EF February 2014 This report is based on research conducted by the Vanderbilt Evidence-based Practice Center (EPC) under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. 290-2007-10065-I). The findings and conclusions in this document are those of the authors, who are responsible for its contents; the findings and conclusions do not necessarily represent the views of AHRQ. Therefore, no statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. The information in this report is intended to help health care decisionmakers—patients and clinicians, health system leaders, and policymakers, among others—make well informed decisions and thereby improve the quality of health care services. -

Trade Mark Journal No
Trade Mark Journal No. 001/2021 31 January, 2021 FEBRUARY 2021 Issue No. 002/2021 1 Trade Mark Journal No. 001/2021 31 January, 2021 CONTENTS General Information 3 Operating Hours 3 Submission of Application 3 Enquiries 3 Trademarks Legislation 4 Forms and Fees 4 INID Codes 5 First Schedule – Fees 7 Second Schedule – Forms 12 Third Schedule – Classification of Goods and Services 14 New Trade Mark Applications Filed 17 Change of Proprietor’s Name 79 Change of Proprietor’s Address 80 Change of Address for Service 83 Subsequent Proprietor Registered 84 Subsequent Proprietor and Address for Service Registered 85 Trade Mark Registered 86 Trade Mark Pending for Renewal (6 months before expiration date) 89 Trade Mark Unpaid Renewal Fee (6 months grace period after expiration date) 108 Trade Mark Renewed 144 Trade Mark Renewed and Change of Agent 145 Trade Mark Renewed and Restored 183 Trade Mark Removed Through Non-Payment of Renewal Fee 184 New Trade Mark Applications Filed Under The Madrid Protocol 185 Trade Mark Registered Filed Under the Madrid Protocol 298 Trade Mark Registered Filed Under the Madrid Protocol due to 18 Months Lapsed 304 Trade Mark Pending for Renewal Filed Under the Madrid Protocol 306 ******* 2 Trade Mark Journal No. 001/2021 31 January, 2021 General Information The Brunei Darussalam Intellectual Property Office (BruIPO) is an Office under the Attorney General’s Chamber and its premises with effect from the 1 April, 2019 and is situated at the following address: Brunei Darussalam Intellectual Property Office (BruIPO) Attorney General’s Chamber Knowledge Hub, Level 2, Anggerek Desa Technology Park Jalan Berakas BB3713, Brunei Darussalam Tel: +673 2380966 Opening Hours With effect from 1 January, 2020, the Brunei Intellectual Property Office (BruIPO) counter will operate as follows: Monday to Thursday : 8.00am to 12.00am 2.00pm to 3.00pm Saturday : 8.00am to 11.00am Friday and Sunday : CLOSED Submission of Applications 1. -
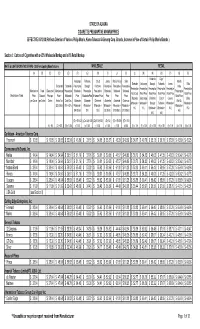
Section I: Cartons of Cigarettes with a 4.5% Wholesale Markup and a 6% Retail Markup
STATE OF ALASKA CIGARETTE PRESUMPTIVE MINIMUM PRICE EFFECTIVE 05/12/08 (Reflects Deletion of Various Philip Morris, Korea Tobacco & Ginseng Corp. Brands, Increase in Price of Certain Philip Morris Brands ) Section I: Cartons of Cigarettes with a 4.5% Wholesale Markup and a 6% Retail Markup WHITE & LIGHT BROWN TAX STAMPS - 2008 Participating Manufacturers WHOLESALE RETAIL (A) (B) (C) (D) (E) (F) (G) (H) (I) (J) (K) (L) (M) (N) (O) (P) (Q) (R) Fairbanks City of Anchorage Fairbanks City of Juneau Mat-Su Valley Sitka Mat-Su Statewide Anchorage Borough Fairbanks Juneau Sitka Discounted Statewide Presumptive Borough Fairbanks Presumptive Presumptive Presumptive Valley Presumptive Presumptive Presumptive Presumptive Presumptive Presumptive Manufacturer Trade Discounted Manufacturer Presumptive Wholesale Presumptive Presumptive Wholesale Wholesale Wholesale Presumptive Retail Cost Retail Price Retail Price Retail Price Retail Price Retail Price Manufacturer / Brand Price Discount Price per Price + Wholesale Price Wholesale Price Wholesale Price Price Price Price Retail Price (Statewide (Anchorage (Fairbanks (City of (Juneau (Sitka per Carton per Carton Carton Alaska Tax Cost (Disc (Statewide (Statewide (Statewide (Statewide (Statewide (Statewide (Mat-Su Wholesale + Wholesale + Borough Fairbanks Wholesale + Wholesale + ($20.00/ctn) Mfr + 4.5%) Wholesale + Wholesale + Wholesale + Wholesale + Wholesale + Wholesale + Wholesale + 6%) 6%) Wholesale + Wholesale + 6%) 6%) $14.02/ctn) 8%) 16%) $3.00/ctn) $10.58/ctn) $10.00/ctn) 6%) 6%) 6%) ((D) + $14.02) (((C)x1.08)+$20 (((C)x1.16)+$20) ((D)+$3) ((D) + $10.58) ((D) + $10) (A) - (B) (C) + $20 (D) x 1.045 x 1.045 ) x 1.045 x 1.045 x 1.045 x 1.045 x1.045 (E) x 1.06 (F) x 1.06 (G) x 1.06 (H) x 1.06 (I) x 1.06 (J) x 1.06 (K) x 1.06 Caribbean - American Tobacco Corp. -

SAWIB09 Program K
12th Annual Stevie® Awards for Women In Business AWARDS BANQUET AND CEREMONIES Friday, November 13, 2015 Marriott Marquis Hotel, New York, New York SPONSOR SILENT AUCTION BENEFICIARY ᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉ ᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉ Welcome to the 2015 Stevie® Awards for Women in Business. We welcome the 400 nominated, amazing women and their guests who’ve gathered tonight for our 12th annual Women in Business Awards dinner. We also welcome the thousands of people around the world who will watch our first-ever Livestream of the awards presentations, and our live “red carpet” pre-show via Periscope. This year’s Stevie Awards for Women in Business received more than 1,400 entries from individuals and organizations in 24 nations. More than 200 professionals around the world participated in the judging process in September-October. The average scores of preliminary judges determined the Finalists. The Gold, Silver and Bronze Stevie Award placements that will be revealed tonight were determined by the members of five final judging committees in late October. All judges are acknowledged in this program. At the conclusion of tonight’s ceremony we’ll present Grand Stevie Award trophies to the five organizations that submitted the best body of entries to this year’s competition — be sure to stay till the end! I invite you to stay abreast of what’s new in the world of Stevie at our web site, StevieAwards.com, and through our presence on Facebook, LinkedIn, and Twitter. Thank you for coming tonight. I know you’ll enjoy yourself! Cordially, Michael Gallagher President The Stevie Awards Program Contents Final Judging Committees 3 Communications & Marketing Awards Categories 15 Preliminary Judges 9 Finalists 13 Company/Organization Awards Categories 17 Media Awards Categories 13 New Product & Service Individual Awards Categories 23 Awards Categories 13 1 ᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉ ᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉᪉ Final Judging Committees Final judging was conducted in October by the members of these five committees. -

Directory by Brand 02-27-06
3/2/2006 Brand Name Manufacturer C/RYO PM/NPM #117 National Tobacco Company RYO NPM 10/20's Dhanraj International C PM 10/20's Dhanraj International RYO PM 1st Choice R.J. Reynolds Tobacco Company C PM 4Aces Top Tobacco, LP RYO PM A Hint of Mint Sherman's 1400 Broadway NYC, Ltd. C PM A Touch of Clove Sherman's 1400 Broadway NYC, Ltd. C PM Ace King Maker Marketing C PM All Star Liberty Brands C PM All-American Value Philip Morris C PM Alpine Philip Morris C PM Always Save Liberty Brands C PM American Bison Wind River Tobacco Company C PM American Bison Wind River Tobacco Company RYO PM American Blend Mac Baren Tobacco Company RYO PM Ashford Von Eicken Group C PM Ashford Von Eicken Group RYO PM Athey Daughters & Ryan, Inc. RYO PM Austin R.J. Reynolds Tobacco Company C PM Bali Peter Stokkebye International, A/S RYO PM Barclay R.J. Reynolds Tobacco Company C PM Bargain Buy R.J. Reynolds Tobacco Company C PM Baron American Blend Farmer's Tobacco Co. of Cynthiana, Inc. C PM Basic Philip Morris C PM Beacon R.J. Reynolds Tobacco Company C PM Belair R.J. Reynolds Tobacco Company C PM Benson & Hedges Philip Morris C PM Best Buy Philip Morris C PM Best Choice Liberty Brands C PM Best Value R.J. Reynolds Tobacco Company C PM Black & Gold Sherman's 1400 Broadway NYC, Ltd. C PM Bonus Value R.J. Reynolds Tobacco Company C PM Boundary VIP Tobacco USA, Ltd. C PM Brand Marketing Liggett Group, Inc. -

Tobacco Cessation Provider Toolkit 2015
Tobacco Cessation Provider Toolkit 2015 Dear Doctor: L.A. Care Health Plan is pleased to provide you with this copy of the Tobacco Cessation Quality Improvement Toolkit. Tobacco use represents an important public health challenge that is preventable. Tobacco use remains the single largest preventable cause of death and disease in the United States. In Los Angeles County, tobacco use claims 8,500 lives annually, tobacco-related diseases cost $4.3 billion dollars each year, and the current smoking prevalence is 14%. Smoking during pregnancy is associated with premature birth, low infant birth weight, miscarriage and complications of pregnancy. Additionally, smokers expose others to secondhand smoke which causes other health problems, including a 20-30% increased risk for lung cancer for non-smokers exposed to secondhand smoke at home. L.A. Care Health Plan is taking an active role in addressing this personal and public health challenge. This toolkit is an example of our efforts to assist you with the evaluation and management of this condition. More effective education, monitoring and treatment of tobacco use can improve patients’ quality of life, and reduce avoidable ER visits and hospitalizations. Evidence based guidelines now recommend initial and annual monitoring of each member for tobacco use. This toolkit offers clinical guidelines and patient education materials to assist you in the care of your members. We hope you find the enclosed guidelines and patient education materials useful. We urge you to utilize the information and resources we have provided and to join us in the tobacco cessation effort. Thank you for joining us in this effort. -

Nevada Directory of Compliant Cigarettes and Roll-Your-Own Brand Families Listed by Manufacturer
INTRODUCTION AND HISTORY OF CHANGES TO THE NEVADA DIRECTORY OF COMPLIANT CIGARETTES AND ROLL-YOUR-OWN BRAND FAMILIES LISTED BY MANUFACTURER The Directory lists all Tobacco Product Manufacturers and brand families that are certified as being in compliance with Nevada law. Only cigarette brands and roll-your-own (RYO) tobacco products listed in the Directory may be sold, offered for sale or possessed for sale in Nevada. A manufacturer listed as a “PM” is a participating manufacturer to the National Tobacco Master Settlement Agreement. A manufacturer listed as an “NPM” is a non-participating manufacturer to the National Tobacco Master Settlement Agreement. The Nevada Department of Taxation, in consultation with the Attorney General, and State Fire Marshall will update the Directory on an as-needed basis to make corrections or to add or delete manufacturers or brand families, and/or or styles. Licensed Nevada distributors will be notified by e- mail, to the address provided to the Department of Taxation, whenever a manufacturer or brand family is added to or removed from the Directory. If you are a licensed Nevada distributor, you are responsible for checking the most recent update of this Directory and any e-mail notifications from the Department of Taxation or Attorney General before you stamp cigarettes or pay the tax on RYO tobacco. Three (3) asterisks *** means that the manufacturer and its brand family or families are listed in the Directory, pending completion of administrative review by the Attorney General. Please note that tobacco products commonly known as “little cigars” currently are not required to be listed in this Directory in order to be lawfully sold in Nevada. -

Intention to Quit Smoking and Polytobacco Use Among College Student Smokers Karen M
University of Kentucky UKnowledge Nursing Faculty Publications College of Nursing 6-2018 Intention to Quit Smoking and Polytobacco Use Among College Student Smokers Karen M. Butler University of Kentucky, [email protected] Melinda J. Ickes University of Kentucky, [email protected] Mary Kay Rayens University of Kentucky, [email protected] Amanda T. Wiggins University of Kentucky, [email protected] Kristin Ashford University of Kentucky, [email protected] See next page for additional authors Right click to open a feedback form in a new tab to let us know how this document benefits oy u. Follow this and additional works at: https://uknowledge.uky.edu/nursing_facpub Part of the Higher Education Commons, and the Preventive Medicine Commons Repository Citation Butler, Karen M.; Ickes, Melinda J.; Rayens, Mary Kay; Wiggins, Amanda T.; Ashford, Kristin; and Hahn, Ellen J., "Intention to Quit Smoking and Polytobacco Use Among College Student Smokers" (2018). Nursing Faculty Publications. 33. https://uknowledge.uky.edu/nursing_facpub/33 This Article is brought to you for free and open access by the College of Nursing at UKnowledge. It has been accepted for inclusion in Nursing Faculty Publications by an authorized administrator of UKnowledge. For more information, please contact [email protected]. Authors Karen M. Butler, Melinda J. Ickes, Mary Kay Rayens, Amanda T. Wiggins, Kristin Ashford, and Ellen J. Hahn Intention to Quit Smoking and Polytobacco Use Among College Student Smokers Notes/Citation Information