Medical Presentations of Psychosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Canadian Stroke Best Practice Recommendations
CANADIAN STROKE BEST PRACTICE RECOMMENDATIONS MOOD, COGNITION AND FATIGUE FOLLOWING STROKE Table 1C: Summary Table for Selected Pharmacotherapy for Post-Stroke Depression Update 2019 Lanctôt KL, Swartz RH (Writing Group Chairs) on Behalf of the Canadian Stroke Best Practice Recommendations Mood, Cognition and Fatigue following Stroke Writing Group and the Canadian Stroke Best Practice and Quality Advisory Committee, in collaboration with the Canadian Stroke Consortium © 2019 Heart & Stroke Heart and Stroke Foundation Mood, Cognition and Fatigue following Stroke Canadian Stroke Best Practice Recommendations Table 1C Table 1C: Summary Table for Selected Pharmacotherapy for Post-Stroke Depression This table provides a summary of the pharmacotherapeutic properties, side effects, drug interactions and other important information on selected classes of medications available for use in Canada and more commonly recommended for post-stroke depression. This table should be used as a reference guide by health care professionals when selecting an appropriate agent for individual patients. Patient compliance, patient preference and/or past experience, side effects, and drug interactions should all be taken into consideration during decision-making, in addition to other information provided in this table and available elsewhere regarding these medications. Selective Serotonin Reuptake Inhibitors (SSRI) Serotonin–norepinephrine reuptake Other inhibitors (SNRI) Medication *citalopram – Celexa *duloxetine – Cymbalta methylphenidate – Ritalin (amphetamine) -

Risk Factors and Outcomes of Rapid Correction of Severe Hyponatremia
Article Risk Factors and Outcomes of Rapid Correction of Severe Hyponatremia Jason C. George ,1 Waleed Zafar,2 Ion Dan Bucaloiu,1 and Alex R. Chang 1,2 Abstract Background and objectives Rapid correction of severe hyponatremia can result in serious neurologic complications, including osmotic demyelination. Few data exist on incidence and risk factors of rapid 1Department of correction or osmotic demyelination. Nephrology, Geisinger Medical Center, Design, setting, participants, & measurements In a retrospective cohort of 1490 patients admitted with serum Danville, , Pennsylvania; and sodium 120 mEq/L to seven hospitals in the Geisinger Health System from 2001 to 2017, we examined the 2 incidence and risk factors of rapid correction and osmotic demyelination. Rapid correction was defined as serum Kidney Health . Research Institute, sodium increase of 8 mEq/L at 24 hours. Osmotic demyelination was determined by manual chart review of Geisinger, Danville, all available brain magnetic resonance imaging reports. Pennsylvania Results Mean age was 66 years old (SD=15), 55% were women, and 67% had prior hyponatremia (last outpatient Correspondence: sodium ,135 mEq/L). Median change in serum sodium at 24 hours was 6.8 mEq/L (interquartile range, 3.4–10.2), Dr. Alexander R. Chang, and 606 patients (41%) had rapid correction at 24 hours. Younger age, being a woman, schizophrenia, lower Geisinger Medical , Center, 100 North Charlson comorbidity index, lower presentation serum sodium, and urine sodium 30 mEq/L were associated Academy Avenue, with greater risk of rapid correction. Prior hyponatremia, outpatient aldosterone antagonist use, and treatment at an Danville, PA 17822. academic center were associated with lower risk of rapid correction. -
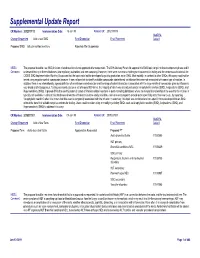
Detail Report
Supplemental Update Report CR Number: 2012319113 Implementation Date: 16-Jan-19 Related CR: 2012319113 MedDRA Change Requested Add a new SMQ Final Disposition Final Placement Code # Proposed SMQ Infusion related reactions Rejected After Suspension MSSO The proposal to add a new SMQ Infusion related reactions is not approved after suspension. The ICH Advisory Panel did approve this SMQ topic to go into the development phase and it Comment: underwent testing in three databases (two regulatory authorities and one company). However, there were numerous challenges encountered in testing and the consensus decision of the CIOMS SMQ Implementation Working Group was that the topic could not be developed to go into production as an SMQ. Most notably, in contrast to other SMQs, this query could not be tested using negative control compounds because it was not possible to identify suitable compounds administered via infusion that were not associated with some type of reaction. In addition, there is no internationally agreed definition of an infusion related reaction and the range of potential reactions associated with the large variety of compounds given by infusion is very broad and heterogenous. Testing was conducted on a set of around 500 terms, the majority of which was already included in Anaphylactic reaction (SMQ), Angioedema (SMQ), and Hypersensitivity (SMQ). It proved difficult to identify potential cases of infusion related reactions in post-marketing databases where the temporal relationship of the event to the infusion is typically not available. In clinical trial databases where this information is more easily available, users are encouraged to provide more specificity about the event, e.g., by reporting “Anaphylactic reaction” when it is known that this event is temporally associated with the infusion. -

Diagnosis and Management of Hyponatremia Melinda Johnson, MD, FHM, FACP, FPHM Associate Professor, Internal Medicine University of Iowa Hospitals and Clinics
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Iowa Research Online UPDATED Diagnosis and Management of Hyponatremia Melinda Johnson, MD, FHM, FACP, FPHM Associate Professor, Internal Medicine University of Iowa Hospitals and Clinics Hyponatremia Low serum Normal or high osmolality serum <280 mOsm/kg osmolality Pseudo- Euvolemic Hypervolemic Redistributive Hypovolemic hyponatremia Una <20 mEq/L Una >20 mEq/L Una >20, Uosm Una >20, Uosm Una<20 mEq/L Una>20 mEq/L Uosm >300 Uosm <100 Hyperlipidemia Hyperglycemia <100 mOsm/kg >300 mOsm/kg mOsm/kg mOsm/kg Mannitol, Drug effect maltose, Advanced renal Hyper- Dehydration (thiazides, ACE- Beer potomania SIADH CHF sucrose, glycine, failure proteinemia I) or sorbitol administration Salt-wasting Psychogenic Diarrhea Postoperative Liver disease Biliary Azotemia nephropathies polydipsia Mineralocorti- Hypo- Nephrotic Alcohol Vomiting coid deficiency thyroidism syndrome intoxication Drug effect Cerebral (thiazides, ACE- sodium-wasting I) Adrenocorticotr opin deficiency Hyponatremia Serum sodium concentration <135 mEq/L Severe hyponatremia <120 mEq/L Disorder of water, not salt Occurs in ~15% of all hospital inpatients Increased morbidity and mortality Symptoms depend on rate of fall 1 Total Body Water Intracellular Interstitial Intravascular •Water load, causing decreased serum Sosm osmolality •Leading to suppressed ADH ADH (vasopressin) •Leading to water excretion in dilute Uosm urine (decreased Uosm) Impaired Renal Water Excretion 1. Inability to -

COVID-19 Mrna Pfizer- Biontech Vaccine Analysis Print
COVID-19 mRNA Pfizer- BioNTech Vaccine Analysis Print All UK spontaneous reports received between 9/12/20 and 22/09/21 for mRNA Pfizer/BioNTech vaccine. A report of a suspected ADR to the Yellow Card scheme does not necessarily mean that it was caused by the vaccine, only that the reporter has a suspicion it may have. Underlying or previously undiagnosed illness unrelated to vaccination can also be factors in such reports. The relative number and nature of reports should therefore not be used to compare the safety of the different vaccines. All reports are kept under continual review in order to identify possible new risks. Report Run Date: 24-Sep-2021, Page 1 Case Series Drug Analysis Print Name: COVID-19 mRNA Pfizer- BioNTech vaccine analysis print Report Run Date: 24-Sep-2021 Data Lock Date: 22-Sep-2021 18:30:09 MedDRA Version: MedDRA 24.0 Reaction Name Total Fatal Blood disorders Anaemia deficiencies Anaemia folate deficiency 1 0 Anaemia vitamin B12 deficiency 2 0 Deficiency anaemia 1 0 Iron deficiency anaemia 6 0 Anaemias NEC Anaemia 97 0 Anaemia macrocytic 1 0 Anaemia megaloblastic 1 0 Autoimmune anaemia 2 0 Blood loss anaemia 1 0 Microcytic anaemia 1 0 Anaemias haemolytic NEC Coombs negative haemolytic anaemia 1 0 Haemolytic anaemia 6 0 Anaemias haemolytic immune Autoimmune haemolytic anaemia 9 0 Anaemias haemolytic mechanical factor Microangiopathic haemolytic anaemia 1 0 Bleeding tendencies Haemorrhagic diathesis 1 0 Increased tendency to bruise 35 0 Spontaneous haematoma 2 0 Coagulation factor deficiencies Acquired haemophilia -

The Pharma-Fever That Almost Got Away
EMERGENCY MEDICINE RESIDENCY CPC The pharma-fever that almost got away XIAO CHI ZHANG, MD, MS; MATTHEW SIKET, MD; WILLIAM BINDER, MD 29 31 EN From the Case Records of the Alpert Medical School of DR. SARAH GAINES: A fever greater than 41.0°C is quite Brown University Residency in Emergency Medicine elevated and unusual. Is this dangerous? What was your differential? DR. XIAO CHI ZHANG: A 68-year-old man was brought into DR. MATTHEW SIKET: Humans generally tolerate tempera- the Emergency Department by his family with chills and tures below 41° C (105.8° F). In contrast to hyperthermia, in altered mental status. Two days prior to his ED presenta- which an imbalance between heat generation versus dissipa- tion, the patient had an episode in which he “spaced-out” tion occurs without up-regulation of the hypothalamic set and was unable to comprehend or acknowledge his wife. She point, fever as a host defense against infection rarely reaches reported that he did not have any signs of seizure activity dangerous levels in neurologically competent individuals. and did not have any focal weakness. The episode lasted Very high temperatures can be related to urosepsis, intraab- approximately 30 minutes and he returned to his baseline. dominal sepsis, C. difficile colitis, meningitis, and central Today he had another episode, but this time associated with venous catheter infections. Hyperpyrexia, defined as tem- chills and rigors. His past medical history was significant perature > 41.5°C (106.7°F) is an uncommon result of infec- for chronic back pain due to bony metastasis from Stage IV tion and usually implies central fever, neurologic malignant non-small cell lung adenocarcinoma, requiring palliative syndrome, malignant hyperthermia, adrenal insufficiency, gamma knife radiation, as well as a daily oral chemotherapy or a drug related cause.1 Our patient was hyperpyrexic, sug- agent, erlotinib, an oral tyrosine kinase inhibitor. -

Faculty Meeting August 9Th, 2011
Review of Systems is a process that includes a review of body systems. It is carried out through a series of questions regarding signs and symptoms. The Review of Systems (ROS) includes information about the following 14 systems. Constitutional: description of general appearance; growth and development, recent weight loss/gain, malaise, chills weakness, fatigue, fever, vital signs, head circumference for a baby, appetite, sleep habits, insomnia, night sweats. Integumentary: (skin and/or breast) rashes, color, sores, dryness, itching, flaking, dandruff, lumps, moles, color change, changes in hair or nails, sweating, hives, bruising, scratches, scars, swelling., acne. Eyes: vision, no change in vision, glasses or contact lenses, last eye exam, eye pain, “eye” redness, excessive tearing, double vision, blurred vision, spots, specks, flashing lights, photophobia, glaucoma, cataracts. Ears, Nose, Mouth/ Throat Ears: hearing loss, tinnitus, vertigo, earaches, ear infections, ear discharges; if hearing is decreased, use of hearing aids. Nose and sinuses: frequent colds, stuffiness’, discharge drainage, nasal itching, hay fever, nosebleeds sinusitis, sinus trouble, sinus pressure, nasal congestion, nasal discharge, nasal infection Mouth/Throat condition of teeth and gums bleeding gums dentures, (how they fit) last dental exam, dry mouth, frequent sore throats, difficulty swallowing, no posterior pharynx pain, hoarseness, sores/ulcers, hoarseness, pyorrhea. Respiratory: cough, sputum, (color, quantity) shortness of breath, pleuritic chest pain, wheezing, asthma, bronchitis, TB, emphysema, pneumonia, hemoptysis, CXR. Cardiovascular: heart trouble; high blood pressure; CV hypertension, heart murmurs, chest pain/ pressure palpitations, dyspnea, orthopnea,, rheumatic fever, paroxysmal nocturnal dyspnea, edema; past EKG or other heart tests. Peripheral Vascular; intermittent claudication, leg cramps, varicose veins, past clots in the vein, syncope, edema. -

2018 National Conference Poster Abstracts
2018 Poster Abstracts August 2-4, 2018 – National Conference of Family Medicine Residents and Medical Students – Kansas City, MO The purpose of the National Conference poster competition is to stimulate research by medical students and family medicine residents, to provide a venue to share innovative and effective educational programs, to showcase unique community projects, and to encourage networking among medical students and residents with similar interests. This year's authors offer valuable information in the categories of clinical inquiry, community projects, educational programs, and research. Poster Presentations Pelvic Inflammatory Disease: Challenges in Diagnosis (Clinical Inquiry) Catherine Peony Khoo, MD University of California, Los Angeles Pelvic inflammatory disease (PID) is a common infection with an estimated lifetime prevalence of 4.4% in reproductive-aged women in the United States. However, given its nonspecific signs and symptoms along with a lack of readily available specific diagnostic testing, PID remains a clinical diagnosis that may pose diagnostic challenges resulting in serious morbidity and mortality. The case presented is that of a perimenopausal woman presenting with fever and lower abdominal pain who was misdiagnosed on multiple occasions in both ambulatory and hospital settings until she was finally found to have sepsis secondary to PID complicated by tuboovarian abscess. Her case highlights multiple important points in diagnosis of PID, pathogenic organisms, and risk factors implicated in PID in postmenopausal women, treatment of PID, and cognitive error contributing to preventable misdiagnosis. How Insufficient Asthma Control Can Break Your Heart (Clinical Inquiry) Louai Naddaf and Sujan Thapaliya, MD Saba University School of Medicine Background: 61-year-old female presented with severe shortness of breath, wheezing, orthopnea, a productive cough, and lower limb edema upon waking up at night. -

PRESCRIBED DRUGS and NEUROLOGICAL COMPLICATIONS K a Grosset, D G Grosset Iii2
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.2004.045757 on 16 August 2004. Downloaded from PRESCRIBED DRUGS AND NEUROLOGICAL COMPLICATIONS K A Grosset, D G Grosset iii2 J Neurol Neurosurg Psychiatry 2004;75(Suppl III):iii2–iii8. doi: 10.1136/jnnp.2004.045757 treatment history is a fundamental part of the healthcare consultation. Current drugs (prescribed, over the counter, herbal remedies, drugs of misuse) and how they are taken A(frequency, timing, missed and extra doses), drugs tried previously and reason for discontinuation, treatment response, adverse effects, allergies, and intolerances should be taken into account. Recent immunisations may also be of importance. This article examines the particular relevance of medication in patients presenting with neurological symptoms. Drugs and their interactions may contribute in part or fully to the neurological syndrome, and treatment response may assist diagnostically or in future management plans. Knowledge of medicine taking behaviour may clarify clinical presentations such as analgesic overuse causing chronic daily headache, or severe dyskinesia resulting from obsessive use of dopamine replacement treatment. In most cases, iatrogenic symptoms are best managed by withdrawal of the offending drug. Indirect mechanisms whereby drugs could cause neurological problems are beyond the scope of the current article—for example, drugs which raise blood pressure or which worsen glycaemic control and consequently increase the risk of cerebrovascular disease, or immunosupressants -

Ekbom Syndrome: a Delusional Condition of “Bugs in the Skin”
Curr Psychiatry Rep DOI 10.1007/s11920-011-0188-0 Ekbom Syndrome: A Delusional Condition of “Bugs in the Skin” Nancy C. Hinkle # Springer Science+Business Media, LLC (outside the USA) 2011 Abstract Entomologists estimate that more than 100,000 included dermatophobia, delusions of infestation, and Americans suffer from “invisible bug” infestations, a parasitophobic neurodermatitis [2••]. Despite initial publi- condition known clinically as Ekbom syndrome (ES), cations referring to the condition as acarophobia (fear of although the psychiatric literature dubs the condition “rare.” mites), ES is not a phobia, as the individual is not afraid of This illustrates the reluctance of ES patients to seek mental insects but rather convinced that they are infesting his or health care, as they are convinced that their problem is her body [3, 4]. This paper deals with primary ES, not the bugs. In addition to suffering from the delusion that bugs form secondary to underlying psychological or physiologic are attacking their bodies, ES patients also experience conditions such as drug reaction or polypharmacy [5–8]. visual and tactile hallucinations that they see and feel the While Morgellons (“the fiber disease”) is likely a compo- bugs. ES patients exhibit a consistent complex of attributes nent on the same delusional spectrum, because it does not and behaviors that can adversely affect their lives. have entomologic connotations, it is not included in this discussion of ES [9, 10]. Keywords Parasitization . Parasitosis . Dermatozoenwahn . Valuable reviews of ES include those by Ekbom [1](1938), Invisible bugs . Ekbom syndrome . Bird mites . Infestation . Lyell [11] (1983), Trabert [12] (1995), and Bak et al. -

Chronic Pain:Useful Terms Personal Injury
Chronic pain:useful terms Personal Injury Analgesic - (also known as a painkiller) is any member of the group of drugs used to relieve pain. Epidural - Epidurals are given for the relief of pain. A cocktail of drugs containing a corticosteriod and a local anaesthetic is injected into the epidural space, between the bone and the membrane that encloses the spinal cord. Fusion - Surgical procedure designed to abolish movement across a joint. Usually involves bone grafting and sometimes metal fixation. Gout - Gout is a type of arthritis that causes sudden and extremely painful inflammatory attacks in the joints – most commonly the big toe, ankles and knees but any other joint too. Guanethidine Blocks – Tourniquet is applied to the limb and guanethidine is injected into a vein to temporarily treat Complex Regional Pain Syndrome symptoms. Hydrotherapy - formerly called hydropathy involves the use of water for pain-relief and treating illness. Hyperalgesia - The perception of a painful stimulus as more painful than normal. Instability - A term used to describe an abnormal increase in the movement of one vertebrae to another. Local Anaesthetic Blocks – Local anaesthetic is injected around the sympathetic nerves from a temporary sympathetic block to treat Complex Regional Pain Syndrome. MRI Scan - Magnetic Resonance Imaging involves a highly technical scanner that uses magnetic fields and computer technology to generate images of the internal anatomy of the body, including discs and nerve roots. It is a painless procedure, although like CT scans, people with claustrophobia may find it difficult. Myelography - A water-soluble, radio-opaque dye is injected into the cerebro-spinal fluid. -

Prognosis of a Case with Paresthesia Associated with Prolonged Touching of an Endodontic Paste to the Inferior Alveolar Nerve
J Clin Exp Dent. 2011;3(Suppl1):e377-81. Inferior alveolar nerve paresthesia. Journal section: Oral Surgery doi:10.4317/jced.3.e377 Publication Types: Case Report Prognosis of a case with paresthesia associated with prolonged touching of an endodontic paste to the inferior alveolar nerve M. Cemil Buyukkurt 1, Hakan Arslan 2, H. Sinan Topcuoglu 2, M. Melih Omezli 3 1 PhD, DDS. Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Sifa University, İzmir, Turkey 2 DDS. Department of Endodontic, Faculty of Dentistry, Ataturk University, Erzurum, Turkey 3 DDS. Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Ataturk University, Erzurum, Turkey Correspondence: Department of Endodontic, Faculty of Dentistry, Ataturk University, Erzurum, 25240, Turkey E-mail address: [email protected] Received: 10/02/2011 Accepted: 08/05/2011 Buyukkurt MC, Arslan H, Topcuoglu HS, Omezli MM. Prognosis of a case with paresthesia associated with prolonged touching of an endodontic paste to the inferior alveolar nerve. J Clin Exp Dent. 2011;3(Suppl1):e377- 81. http://www.medicinaoral.com/odo/volumenes/v3iSuppl1/jcedv3iSu- ppl1p377.pdf Article Number: 50506 http://www.medicinaoral.com/odo/indice.htm © Medicina Oral S. L. C.I.F. B 96689336 - eISSN: 1989-5488 eMail: [email protected] Abstract Paresthesia is described as an abnormal sensation, such as burning, pricking, tickling, tingling, formication or num- bness. Several conditions can cause paresthesia. This article presents a case of paresthesia caused by the extrusion of endodontic paste (Endomethasone®) into the mandibular canal. The clinical manifestations comprised the numb- ness on the right side of the mandible and right lower lip, appearing after endodontic treatment.