A Dissertation on COMPARISON of MIFEPRISTONE with FOLEY's CATHETER for INDUCTION of LABOUR in POST DATED PREGNANCY
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
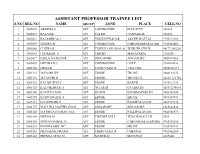
Assistant Professor Trainee List
ASSISTANT PROFESSOR TRAINEE LIST S.NO REG.NO NAME GROUP ZONE PLACE CELL.NO 1 5005002 ABARNA.S APT COIMBATORE POLLACHI 288830 2 5005027 BALAJI.K APT SALEM NAMAKKAL 252624 3 5005031 BALAMBIGA.J APT VIRUDHUNAGAR ARUPPUKOTTAI 9790311169 4 5005059 CHITRA .R APT COIMBATORE CHENNIAMPALAYAM 9942602460 5 5005060 CHITRA.R APT THIRUVANNAMALAI THIRUPPATHUR 04177 243224 6 5005061 CHOKKAR .N APT TRICHY MANAPARAI 2260206 7 5005077 EZHLILNATHAN.R APT SINGAPORE SINGAPORE 9443079960 8 5005085 GEETHA.K.J. APT COIMBATORE OOTY 9942028536 9 5005100 HEMA.K APT THIRUVALLUR VELLORE 99940 63717 10 5005111 JAYASRI .KP APT ERODE ERODE 9442122278 11 5005116 JEYANTHI.D APT ERODE ERODE DT. 04285-221702 12 5005123 KALIMUTHU.V APT ERODE KARUR 9994311315 13 5005125 KALVIKARASI .S APT GUJARAT GUJARATH 0265-3298695 14 5005140 KAVITHA.M.M. APT HOSUR DHARMAPURI-DT. 9486358784 15 5005155 KUPPUSWAMI .A APT ERODE ERODE 9843269931 16 5005161 LOGESWARI.S APT ERODE PALLIPALAYAM 9487927370 17 5005177 MALLIKA MATHIVANAN APT SINGAPORE SINGAPORE 6565643834 18 5005188 MATHIVATHANAN .M.K APT ERODE PALLIPALAYAM. 9842988871 19 5005190 MEENA.M APT TIRUNELVELI THACHANALLUR 5655 20 5005196 MOHANAMBAL .G APT ERODE CHENNIMALAI-ERODE 9965360200 21 5005239 PONNUSAMY .VC APT ERODE ERODE 9965769357 22 5005242 PRAVEENKUMAR.R APT THIRUVALLUR CHENNAI 9962062264 23 5005245 PREMALATHA.M APT MADURAI MADURAI 2629604 24 5005254 RAJALAKSHMI.N APT SALEM NAMAKKAL 9442156240 25 5005280 RUPA.T APT THIRUVANNAMALAI THIRUVANNAMALAI 7845443323 26 5005285 SANGILIKALAI .M.V APT DINDIGUL BODI 283105 27 5005286 SANKAR -

ANNEXURE 5.8 (CHAPTER V , PARA 25) FORM 9 List of Applications For
ANNEXURE 5.8 (CHAPTER V , PARA 25) FORM 9 List of Applications for inclusion received in Form 6 Designated location identity (where Constituency (Assembly/£Parliamentary): Thiruporur Revision identity applications have been received) 1. List number@ 2. Period of applications (covered in this list) From date To date 01/11/2020 01/11/2020 3. Place of hearing * Serial number$ Date of receipt Name of claimant Name of Place of residence Date of Time of of application Father/Mother/ hearing* hearing* Husband and (Relationship)# 1 01/11/2020 AMUTHA PRABAKARAN (H) DOOR NO 2/70, INDIRA GANDHI STREET, KARANAI- VILLAGE,CHENNAI, , 2 01/11/2020 Kishore Kumar Thakur Indra Kant Thakur (F) Flat No. D02 303, Provident Cosmo City 41 Dr Abdul Kalam Road, Pudupakkam, , 3 01/11/2020 VIMALRAJ E ELUMALAI (F) 3/327, CSI SCHOOL STREET, KAYAR, , 4 01/11/2020 JAYARAMAN V VENKATESAN 113, bigstreet, NATHAM VENKATESAN M VENKATESAN (F) ,NATHAM KARIYACHERI, , £ In case of Union territories having no Legislative Assembly and the State of Jammu and Kashmir Date of exhibition at @ For this revision for this designated location designated location under Date of exhibition at Electoral * Place, time and date of hearings as fixed by electoral registration officer rule 15(b) Registration Officer¶s Office under $ Running serial number is to be maintained for each revision for each designated rule 16(b) location # Give relationship as F-Father, M=Mother, and H=Husband within brackets i.e. (F), (M), (H) 17/12/2020 ANNEXURE 5.8 (CHAPTER V , PARA 25) FORM 9 List of Applications for inclusion received in Form 6 Designated location identity (where Constituency (Assembly/£Parliamentary): Thiruporur Revision identity applications have been received) 1. -
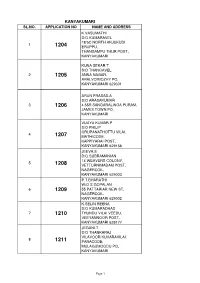
Kanyakumari Sl.No
KANYAKUMARI SL.NO. APPLICATION NO NAME AND ADDRESS K.VASUMATHI D/O KUMARAVEL 18/5C,NORTH ANJUKUDI 1 1204 ERUPPU, THANGAMPU THUR POST, KANYAKUMARI KUNA SEKAR.T S/O THANGAVEL 2 1205 ANNA NAGAR, ARALVOIMOZHY PO, KANYAKUMARI 629301 ARUN PRASAD.A S/O ARASAKUMAR 3 1206 4.55R SANGARALINGA PURAM, JAMES TOWN PO, KANYAKUMARI VIJAYA KUMAR.P S/O PHILIP ORUPANATHOTTU VILAI, 4 1207 MATHICODE, KAPPIYARAI POST, KANYAKUMARI 629156 JEEVA.S D/O SUBRAMANIAN 14 WEAVERS COLONY, 5 1208 VETTURNIMADAM POST, NAGERCOIL, KANYAKUMARI 629003 P.T.BHARATHI W/O S.GOPALAN 6 1209 55 PATTARIAR NEW ST, NAGERCOIL, KANYAKUMARI 629002 K.SELIN REENA D/O KUMARADHAS 7 1210 THUNDU VILAI VEEDU, VEEYANNOOR POST, KANYAKUMARI 629177 JEGANI.T D/O THANKARAJ VILAVOOR KUVARAVILAI, 8 1211 PARACODE, MULAGUMOODU PO, KANYAKUMARI Page 1 FELSY FREEDA.L D/O LAZER 9 1212 MALAANTHATTU VILAI, PALLIYADI POST, KANYAKUMARI 629169 CHRISTAL KAVITHA. R D/O RAJAN 10 1213 VALIYAVILAGAM HOUSE ST, MANKADU POST, KANYAKUMARI 629172 PRABHA.P D/O PADMANABHAN 11 1214 EATHENKADU, FRIDAYMARKET POST, KANYAKUMARI 629802 KALAI SELVI.N D/O NARAYANA PERUMAL 12 1215 THERIVILAI SWAMITHOPPU POST, KANYAKUMARI 629704 NAGALAKSHMI.M D/O MURUGESAN LEKSHMI BHAVAN, 13 1216 CHAKKIYANCODE, NEYYOOR POST, KANYAKUMARI 629802 BEULA.S D/O SATHIADHAS 14 1217 ANAN VILAI, KEEZHKULAM PO, KANYAKUMARI 629193 MAHESWARI.S D/O SIVACHANDRESWARAN 15 1218 1/20BTHEKKURICHI, RAJAKKAMANGALAM POST, KANYAKUMARI 629503 PREMALATHA.S W/O MURALIRAJ V.L, 460F-1, M.S.ROAD, 16 1219 SINGARATHOPPUPAR, VATHIPURAM, NAGERCOIL, KANYAKUMARI 629003 SUBASH.T S/O THANKAPPAN MANALI KATTU VILAI, 17 1220 PUTHEN VEEDU THICKA, NAMCODE PO, KANYAKUMARI 629804 Page 2 J. -
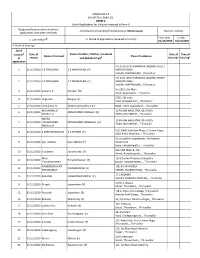
ANNEXURE 5.8 (CHAPTER V, PARA 25) FORM 9 List of Applica Ons For
ANNEXURE 5.8 (CHAPTER V, PARA 25) FORM 9 List of Applicaons for inclusion received in Form 6 Designated locaon identy (where Constuency (Assembly/£Parliamentary): Maduravoyal Revision identy applicaons have been received) From date To date @ 2. Period of applicaons (covered in this list) 1. List number 01/12/2020 01/12/2020 3. Place of hearing* Serial $ Date of Name of Father / Mother / Husband Date of Time of number Name of claimant Place of residence of receipt and (Relaonship)# hearing* hearing* applicaon NO 27/A, DEVI NARAYANA LAKSHMI STREET 1 01/12/2020 C E PRAKASAN C E NARAYANAN (F) MARUTHI RAM NAGAR, AYAPPAKKAM, , Thiruvallur NO 27/A, DEVI NARAYANA LAKSHMI STREET 2 01/12/2020 C E PRAKASAN C E NARAYANAN (F) MARUTHI RAM NAGAR, AYAPPAKKAM, , Thiruvallur No 2319, 5th Main 3 01/12/2020 Sumathi R Ramesh (H) Road, Ayapakkam, , Thiruvallur 2785, 5th main 4 01/12/2020 Jaiganesh Rangan (F) road, Ayyappakkam, , Thiruvallur 5 01/12/2020 Hemalatha D Dhatshinamoorthy K (H) 8640, TNHB, Ayapakkam, , Thiruvallur MOHAMMED 10 FA JAIN NAKSHTRA, 86 UNION 6 01/12/2020 MOHAMMED NORULLA (F) NASRULLA ROAD, NOLAMBUR, , Thiruvallur HAJIRA 10 FA JAIN NAKSHTRA, 86 UNION 7 01/12/2020 MOHAMMED MOHAMMED NASRULLA (H) ROAD, NOLAMBUR, , Thiruvallur NASRULLA C16, DABC Gokulam Phase 3, Sriram Nagar 8 01/12/2020 S SURIYAKRISHNAN K S SATHISH (F) Main Road, Nolambur, , Thiruvallur 42 sri Lakshmi apartments, 3rd Avenue 9 01/12/2020 sijo mathew biju mathew (F) millennium town, adayalampau, , Thiruvallur 312 VNR Milford, 7th 10 01/12/2020 Sundaram savarimuthu (F) Street, -
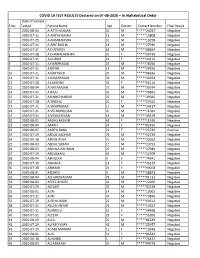
COVID 19 TEST RESULTS Declared on 07-08-2020
COVID 19 TEST RESULTS Declared on 07-08-2020 – In Alphabetical Order Date of Sample S No Tested Patient Name Age Gender Contact Number Final Result 1 2020-08-04 A AJITH KUMAR 22 M *****24027 Negative 2 2020-07-31 A AMITH BASHA 18 M *****15808 Negative 3 2020-07-25 A ANSAR BASHA 33 M *****16258 Negative 4 2020-07-31 A ARIF BASHA 18 M *****27930 Negative 5 2020-07-31 A D JEYAVEL 63 M *****08694 Negative 6 2020-07-28 A DHANALAKSHMI 30 F *****90739 Negative 7 2020-07-31 A DURGA 21 F *****24215 Negative 8 2020-07-31 A HARIPRASAD 29 M *****78756 Negative 9 2020-07-31 A IRFAN 18 M *****24556 Negative 10 2020-07-31 A KARTHICK 30 M *****08626 Negative 11 2020-07-31 A KAVIARASAN 36 M *****44033 Negative 12 2020-07-28 A LAKSHMI 32 F *****66116 Negative 13 2020-08-04 A NATARAJAN 73 M *****30974 Negative 14 2020-07-31 A RAJU 33 M *****49861 Negative 15 2020-07-31 A RAMCHANDAR 33 M *****49045 Negative 16 2020-07-28 A SINDHU 20 F *****27610 Negative 17 2020-07-31 A SIVAPRASAD 19 M *****30427 Negative 18 2020-07-31 A VELMURUGAN 40 M *****71042 Negative 19 2020-07-31 A VENKATESAN 18 M *****39579 Negative 20 2020-08-01 AADHILAKSHMI 48 F *****11976 Negative 21 2020-08-04 AARTHI 24 F *****82450 Negative 22 2020-08-07 AASIFA BANU 22 F *****25720 Positive 23 2020-07-29 ABDUL KADHAR 78 M *****52729 Negative 24 2020-06-18 ABDUL RHIM 70 M *****25092 Negative 25 2020-08-03 ABDUL SUBAN 15 M *****23753 Negative 26 2020-08-03 ABDULA RAHMAN 20 M *****47984 Negative 27 2020-07-29 ABDULKANI 65 M *****12709 Negative 28 2020-08-04 ABHILEKA 9 F *****74941 Negative 29 2020-07-30 -

Chennai Tla Hearing Board
TLA HEARING BOARD Hearing Schedule from 05/10/2015 to 20/10/2015 Location: CHENNAI S.No. TM No. Class Hearing Date Hearing Schedule Proprietor Name Agent Name 1 2136304 45 10/5/2015 Morning (10.30 am to BRB. OLIMUTHU A. ARIVAZHAGAN 1.00 pm) 2 2136308 99 10/5/2015 Morning (10.30 am to UNIVERSITA COMMERCIALE LUIGI DEPENNING & DEPENNING 1.00 pm) BOCCONI 3 2088437 29 10/5/2015 Morning (10.30 am to NATURO FOOD & FRUIT PRODCUTS APR ASSOCIATES. 1.00 pm) PVT. LTD 4 2091509 11 10/5/2015 Morning (10.30 am to THERMEN HEATING TECHNOLOGIES MAKHIJA & ASSOCIATES. 1.00 pm) PVT. LTD 5 2097485 5 10/5/2015 Morning (10.30 am to RADHA RAMANA HEALTH CARE PVT R.K. DEWAN AND COMPANY 1.00 pm) LTD 6 2097486 5 10/5/2015 Morning (10.30 am to AVA DUTTA MARKETING PVT LTD. R.K. DEWAN AND COMPANY 1.00 pm) 7 2105156 99 10/5/2015 Morning (10.30 am to GREYNIUM INFORMATION INDUS LAW 1.00 pm) TECHNOLOGIES PRIVATE LTD. 8 2105157 99 10/5/2015 Morning (10.30 am to GREYNIUM INFORMATION INDUS LAW 1.00 pm) TECHNOLOGIES PRIVATE LTD. 9 2106315 5 10/5/2015 Morning (10.30 am to M.HEMALATHA INPRO TRADE MARK SERVICES. 1.00 pm) 10 815599 19 10/5/2015 Morning (10.30 am to PALLAVA GRANITE INDUSTRIES A. PRABHAKARA REDDY. 1.00 pm) (INDIA) LTD 11 1518296 41 10/5/2015 Morning (10.30 am to BASHEERUDDIN BABUKHAN I-WIN IP SERVICES 1.00 pm) 12 2017784 99 10/5/2015 Morning (10.30 am to SAMI LABS LIMITED SAMI LABS LIMITED 1.00 pm) 13 2032031 33 10/5/2015 Morning (10.30 am to SPR GROUP HOLDINGS PVT. -
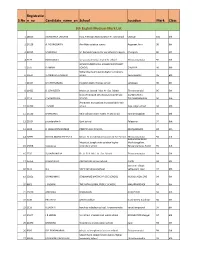
Mark List.Xlsx
Registratio S.No n_no Candidate_name_enSchool Location Mark Class 6th English Medium Mark List 1 18025 AISHWARYA LAKSHMI Indo American Matriculation Hr. Sec school Cheyyar 100 6th 2 24128 A. POONGGAVIN Aim Matriculation school Aagaram, Arni 98 6th 3 21945 S.MOKESH Sri Ramakrishna mat hr sec school chengam Chengam 96 6th 4 5177 RAKSHANA S sri saraswathi vikas matric hr.school Thiruvannamalai 96 6th GOVERNMENT GIRLS HIGHER SECONDARY 5 1575 R AMRIN SCHOOL CHEYYAR 96 6th Sidhardha matriculation higher secondary 6 20514 V.AMIRTHA VARSHINI school Peranamallur 96 6th 7 11354 M.DHEENABANU Kingston matric he.Dec.school vandavasi 96 6th 8 11422 D. JOY BLESSY Mount st. Joseph. Mat. Hr. Sec. School Tiruvannamalai 96 6th GANDHI NAGAR MATRICULATION HR SEC KIZHNACHIPET 9 3214 C M MOSHIHA SCHOOL TIRUVANNAMALAI 96 6th Amravathi murugaiyan municipal girls high 10 11700 T.SALINI school Raja ranjan street 94 6th 11 13130 DHARSAN S Vela vidhyasharam matric hr sec school Kannamangalam 94 6th 12 15334 priyadarshini k Govt school Palayanur 94 6th 13 4228 R. JASHVANTH KUMAR PINKZ PUBLIC SCHOOL KASTHAMBADI 94 6th 14 14991 NICOLA MARIA GREENE.N Mount.St.Joseph.Matriculation.Hr.Sec.School Thiruvannamalai 94 6th Avalurpettai Road, Mount.st.Joseph.matriculation higher Mathalangulam, 15 13858 S.Anusuya secondary school Tiruvannamalai, Tamil 94 6th 16 2535 SUHAIBU NISHA Dr. V. G. N. Mat. Hr. Sec. School Thiruvannamalai 94 6th 17 16354 R.SHIVARAM. AIM MATRIC Hr.Sec.School. ARANI 94 6th keeranur village, 18 9111 G.S SKV International school vettavalam road 94 6th 19 32051 SRIKRISHNA S SENDHAMIZ MATRIC HR SEC SCHOOL KILKODUNGALORE 94 6th 20 8861 J.YAZHINI THE PATH GLOBAL PUBLIC SCHOOL MALAPPAMBADI 94 6th 21 15230 ANUSHKA BHAGAVAN KILNATHUR 94 6th 22 3236 PRATAP.R GHSS Korakkai Road street, Korakkai 94 6th 23 1116 V.SACHIN kendriya vidyalaya school, Tiruvannamalai kanathampoondi 94 6th 24 14347 J R SANJAI Virutcham international public school ARUGAVOOR,cheyyar 94 6th 25 21090 MONISHA St.Antony's matriculation school Kanji 94 6th 26 9920 R.P. -

“A Cross Sectional Study on Age at Menarche Among the Women with Operative and Normal Delivery”
“A CROSS SECTIONAL STUDY ON AGE AT MENARCHE AMONG THE WOMEN WITH OPERATIVE AND NORMAL DELIVERY” Dissertation submitted to The Tamilnadu DR. M.G.R. Medical University In partial fulfilment of the requirement for the award of the degree of M.S OBSTETRICS AND GYNAECOLOGY BRANCH II The Tamilnadu DR. M.G.R. Medical University, Institute of Obstetrics and Gynaecology, Government Hospital for Women & Children, Madras Medical College, MAY- 2018 BONAFIDE CERTIFICATE This is to certify that this dissertation titled “A cross sectional study on age at menarche among the women with operative and normal delivery” is the bonafide original work done by Dr. SINDHU. S Postgraduate in the department of Obstetrics and Gynaecology, Under the guidance of Dr. Usharani MD, DGO., Professor, Institute of Obstetrics and Gynaecology, Government Hospital for Women and Children, Madras Medical College, Chennai, towards partial fulfilment of the requirement of the Tamilnadu DR. M.G.R. Medical University for the award of M.S Degree in Obstetrics and Gynaecology, March 2018. The period of post graduate study is from May 2015 to May2018. Prof. Dr. Usharani MD, DGO Prof. Dr. D. Tamil selvi MD., DGO., Professor, Director, Institute of obstetrics Institute of Social Obstetrics, and Gynaecology, Kasturba Gandhi hospital, Madras medical college, Madras medical college Chennai -600005. Chennai -600005 Prof. Dr. R. Narayanababu MD., DCh., Dean, Madras Medical College, Chennai- 600003. DECLARATION I solemnly declare that this dissertation “A cross sectional study on age at menarche among the women with operative and normal delivery” was prepared by me under the guidance and supervision of Dr. -

Tamil Nadu Siddha Medical Council, Chennai - 106
TAMIL NADU SIDDHA MEDICAL COUNCIL, CHENNAI - 106. REGISTER OF 2D BARCODE ISSUED REGISTERED SIDDHA MEDICAL PRACTITIONERS UPTO 31st MARCH 2017 (Ref No. 60/TNSMC/2017.) A - Class No. VI(1)/330/2017. Sl.No. Reg. No. Name Father's Name Qualification Date of Regn. Address (1) (2) (3) (4) (5) (6) (7) ARIYALUR DISTRICT 1 4608 Dr. AISHWARYA A K. Ambalavanan B.S.M.S. 22/06/2015 No.1334, Palani Andavar Koil Street, Vilandai, Andimadam, Ariyalur - 621801 2 3316 Dr. AKHILA DEVI S P S. Panneer Selvam B.S.M.S. 20/05/2009 I-26-A, Anumar Koil Street, T.Palur, Ariyalur - 612904 M.D.(S) 21/07/2016 3 3993 Dr. AKILA K R. KALIYAPERUMAL B.S.M.S. 24/05/2012 No.1/89, North Street, Sirukalathur Post, Sendurai Taluk, M.D.(S) 14/03/2016 Ariyalur - 621710 4 4693 Dr. AMALA M V. Manickam B.S.M.S. 20/07/2015 No.3/759-3/154, West Street, Mathumadakki, Keezhamaligai Post, Sendurai Taluk, Ariyalur - 621710 5 3903 Dr. ANANTHI K V K. Vaithianathan B.S.M.S. 13/07/2011 No.112, Main Road, Variyankaval, Udayarpalayam Taluk, 2 M.D.(S) 27/01/2015 Ariyalur - 621806 6 4137 Dr. ANBARASI A S. Anbalagan B.S.M.S. 22/07/2013 No.123, Ponparappi / Kudikadu Post, Sendurai Taluk, M.D.(S) 14/03/2017 Ariyalur - 621710 7 4841 Dr. ANILADEVI P S. PANNEER SELVAM B.S.M.S. 07/01/2016 No.1/26, Anumar Koil Street, T. Palur, Ariyalur - 612904 8 4399 Dr. ANITHA A M. -

National Day Awards 2020
1 NATIONAL DAY AWARDS 2020 THE ORDER OF TEMASEK (WITH HIGH DISTINCTION) [Darjah Utama Temasek (Dengan Kepujian Tinggi)] Name Designation 1 Prof S Jayakumar Senior Legal Adviser to the Minister for Foreign Affairs 1 2 THE DISTINGUISHED SERVICE ORDER [Darjah Utama Bakti Cemerlang] Name Designation 1 Mr Koh Choon Hui Chairman, Singapore Children’s Society 2 Prof Wang Gungwu Former Chairman, ISEAS – Yusof Ishak Institute Former Chairman, Lee Kuan Yew School of Public Policy, National University of Singapore Former Chairman, East Asian Institute, National University of Singapore 2 3 THE MERITORIOUS SERVICE MEDAL [Pingat Jasa Gemilang] Name Designation 1 Ms Chan Lai Fung Permanent Secretary (National Research & Development) Permanent Secretary (Public Sector Science and Technology Policy and Plans Office) Chairman, A*STAR 2 Assoc Prof Benjamin Ong Kian Chung Immediate Past Director of Medical Services 3 4 THE PUBLIC SERVICE STAR (BAR) [Bintang Bakti Masyarakat (Lintang)] Name Designation Aljunied GRC 1 Mr Tng Kay Lim, BBM Chairman, Paya Lebar CCC Bishan-Toa Payoh GRC 2 Mr Roland Ng San Tiong, JP, BBM Chairman, Toa Payoh Central CCC East Coast GRC 3 Mdm Susan Ang Siew Lian, BBM Treasurer, Changi Simei CCC Holland-Bukit Timah GRC 4 Mr Lim Cheng Eng, BBM Patron, Bukit Timah CCMC Jurong GRC 5 Mr Richard Ong Chuan Huat, BBM Chairman, Bukit Batok East CCC 6 Mr Victor Liew Cheng San, BBM Vice-Chairman, Taman Jurong CCC Marine Parade GRC 7 Mr Ong Pang Aik, BBM Patron, Braddell Heights CCMC Sembawang GRC 8 Mr Norman Aw Kai Aik, BBM Chairman, Canberra -

Tamil Nadu Government Gazette
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2015 [Price: Rs. 32.80 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 12] CHENNAI, WEDNESDAY, MARCH 25, 2015 Panguni 11, Jaya, Thiruvalluvar Aandu – 2046 Part VI—Section 4 Advertisements by private individuals and private institutions CONTENTS PRIVATE ADVERTISEMENTS Pages. Change of Names .. 841-921 Notice .. 922 NOTICE NO LEGAL RESPONSIBILITY IS ACCEPTED FOR THE PUBLICATION OF ADVERTISEMENTS REGARDING CHANGE OF NAME IN THE TAMIL NADU GOVERNMENT GAZETTE. PERSONS NOTIFYING THE CHANGES WILL REMAIN SOLELY RESPONSIBLE FOR THE LEGAL CONSEQUENCES AND ALSO FOR ANY OTHER MISREPRESENTATION, ETC. (By Order) Director of Stationery and Printing. CHANGE OF NAMES 12112. I, E. Kalaiarasi, wife of Thiru K. Ekambaram, 12115. I, Fathima Zohra Salim, wife of Thiru born on 4th December 1981 (native district: Kancheepuram), A. Saleem, born on 18th June 1972 (native district: Chennai), residing at No. 3/432, Rajiv Gandhi Salai, Mettukuppam, residing at No. 78, (A2 Vadhumathi) T.P.K. Road, O.M.R., Chennai-600 097, shall henceforth be Andalpuram, Agrini Apartment, Madurai-625 003, shall known as E. KALAIARASSI. henceforth be known as FATHIMA ZOHRA. E. KALAIARASI. Chennai, 17th March 2015. FATHIMA ZOHRA SALIM. Madurai, 16th March 2015. 12113. I, B. Khudbunissa Jeelani, daughter of Thiru A. Basheer Ahmed, born on 28th July 1967 (native 12116. My daughter, G.K. Eeshvi, born on 3rd April 2014 district: Coimbatore), residing at Old No. 57, New No. 53, (native district: Madurai), residing at No. 7-3, Manimegalai Raja Street, Coimbatore-641 001, shall henceforth Street, Avaniapuram, Ganapathy Nagar, Madurai-625 012, be known as M. -
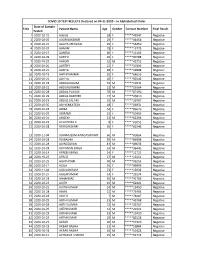
COVID 19 TEST RESULTS Declared on 04-11-2020.Xlsx
COVID 19 TEST RESULTS Declared on 04-11-2020 – In Alphabetical Order Date of Sample S No Patient Name Age Gender Contact Number Final Result Tested 1 2020-10-21 Aabida 28 F *****43947 Negative 2 2020-10-29 AADHILAKSHMI 29 F *****04458 Negative 3 2020-10-21 AALIYA MEGATAJ 25 F *****56450 Negative 4 2020-10-29 AAMIAH 28 F *****13773 Negative 5 2020-10-19 AANDAL 60 F *****13166 Negative 6 2020-10-29 AARIFA 26 F *****90788 Negative 7 2020-10-29 AARON 32 M *****42752 Negative 8 2020-10-20 AARTHY 22 F *****30309 Negative 9 2020-10-21 AASIYA 28 F *****42808 Negative 10 2020-10-19 AATHIYAMMAL 55 F *****64616 Negative 11 2020-10-21 AATIYA 10 F *****92542 Negative 12 2020-10-23 ABDUALKALIM 59 M *****19541 Negative 13 2020-10-21 ABDUALRAHIM 21 M *****55964 Negative 14 2020-10-28 ABDUL PUDUSH 70 M *****67190 Negative 15 2020-10-29 ABDUL RASHEED 74 M *****25810 Negative 16 2020-10-23 ABDUL SALAM 25 M *****29707 Negative 17 2020-10-30 ABI VENKATESN 18 F *****06931 Negative 18 2020-10-23 ABIBA 54 F *****86676 Negative 19 2020-10-29 ABIRAMI 23 F *****30309 Negative 20 2020-10-21 ABISEKH 23 M *****62294 Negative 21 2020-10-23 ACHI PRIYA K 8 F *****61052 Negative 22 2020-10-28 ADHILAKSHMI 66 F *****81546 Negative 23 2020-11-04 ADHIMULAM RANGASWAMY 60 M *****80864 Negative 24 2020-10-28 ADIBASHA 39 M *****86908 Negative 25 2020-10-28 ADINESAVAN 41 M *****09633 Negative 26 2020-10-28 ADVANAN SINGH 24 M *****96443 Negative 27 2020-10-14 AFREEN BANU 24 F *****11723 Negative 28 2020-10-25 AFROZ 27 M *****12261 Negative 29 2020-10-29 AGATHIYAN 30 M *****06253