1 Convergence and Divergence in Anti-Predator Displays: a Novel Approach to Quantitative
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Reptiles (Viviparous Lizards and Slow-Worms Anguis REFERENCES Fragilis)
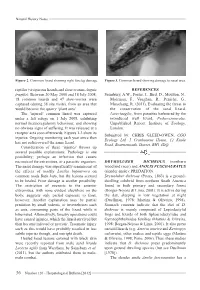
Natural History Notes Figure 2. Common lizard showing right foreleg damage. Figure 3. Common lizard showing damage to nasal area. reptiles (viviparous lizards and slow-worms Anguis REFERENCES fragilis). Between 20 May 2008 and 18 July 2008, Sainsbury, A.W., Foster, J., Bird, D., Moulton, N., 18 common lizards and 47 slow-worms were Molenaar, F., Vaughan, R., Peniche, G., captured (during 38 site visits), from an area that Marschang, R. (2011). Evaluating the threat to would become the quarry ‘plant area’. the conservation of the sand lizard, The ‘injured’ common lizard was captured Lacertaagilis, from parasites harboured by the under a felt refuge on 1 July 2008, exhibiting introduced wall lizard, Podarcismuralis. normal thermoregulatory behaviour, and showing Unpublished Report. Institute of Zoology, no obvious signs of suffering. It was released at a London. receptor area soon afterwards. Figures 1-3 show its Submitted by: CHRIS GLEED-OWEN, CGO injuries. Ongoing monitoring each year since then Ecology Ltd, 5 Cranbourne House, 12 Knole has not rediscovered the same lizard. Road, Bournemouth, Dorset, BH1 4DQ. Consideration of these ‘injuries’ throws up several possible explanations. Pathology is one possibility; perhaps an infection that causes necrosis of the extremities, or a parasitic organism. DRYMOLUBER DICHROUS (northern The nasal damage was superficially reminiscent of woodland racer) and ANOLIS FUSCOAURATUS the effects of toadfly Lucilia bufonivora on (slender anole): PREDATION. common toads Bufo bufo, but the lesions seemed Drymoluber dichrous (Peters, 1863) is a ground- to be healed. Frost damage is another possibility. dwelling colubrid from northern South America The restriction of necrosis to the anterior found in both primary and secondary forest extremities, with none evident elsewhere on the (Borges-Nojosa & Lima, 2001). -

Xenosaurus Tzacualtipantecus. the Zacualtipán Knob-Scaled Lizard Is Endemic to the Sierra Madre Oriental of Eastern Mexico
Xenosaurus tzacualtipantecus. The Zacualtipán knob-scaled lizard is endemic to the Sierra Madre Oriental of eastern Mexico. This medium-large lizard (female holotype measures 188 mm in total length) is known only from the vicinity of the type locality in eastern Hidalgo, at an elevation of 1,900 m in pine-oak forest, and a nearby locality at 2,000 m in northern Veracruz (Woolrich- Piña and Smith 2012). Xenosaurus tzacualtipantecus is thought to belong to the northern clade of the genus, which also contains X. newmanorum and X. platyceps (Bhullar 2011). As with its congeners, X. tzacualtipantecus is an inhabitant of crevices in limestone rocks. This species consumes beetles and lepidopteran larvae and gives birth to living young. The habitat of this lizard in the vicinity of the type locality is being deforested, and people in nearby towns have created an open garbage dump in this area. We determined its EVS as 17, in the middle of the high vulnerability category (see text for explanation), and its status by the IUCN and SEMAR- NAT presently are undetermined. This newly described endemic species is one of nine known species in the monogeneric family Xenosauridae, which is endemic to northern Mesoamerica (Mexico from Tamaulipas to Chiapas and into the montane portions of Alta Verapaz, Guatemala). All but one of these nine species is endemic to Mexico. Photo by Christian Berriozabal-Islas. amphibian-reptile-conservation.org 01 June 2013 | Volume 7 | Number 1 | e61 Copyright: © 2013 Wilson et al. This is an open-access article distributed under the terms of the Creative Com- mons Attribution–NonCommercial–NoDerivs 3.0 Unported License, which permits unrestricted use for non-com- Amphibian & Reptile Conservation 7(1): 1–47. -

HERPETOLOGICAL BULLETIN Number 106 – Winter 2008
The HERPETOLOGICAL BULLETIN Number 106 – Winter 2008 PUBLISHED BY THE BRITISH HERPETOLOGICAL SOCIETY THE HERPETOLOGICAL BULLETIN Contents RESEA R CH AR TICLES Use of transponders in the post-release monitoring of translocated spiny-tailed lizards (Uromastyx aegyptia microlepis) in Abu Dhabi Emirate, United Arab Emirates Pritpal S. Soorae, Judith Howlett and Jamie Samour .......................... 1 Gastrointestinal helminths of three species of Dicrodon (Squamata: Teiidae) from Peru Stephen R. Goldberg and Charles R. Bursey ..................................... 4 Notes on the Natural History of the eublepharid Gecko Hemitheconyx caudicinctus in northwestern Ghana Stephen Spawls ........................................................ 7 Significant range extension for the Central American Colubrid snake Ninia pavimentata (Bocourt 1883) Josiah H. Townsend, J. Micheal Butler, Larry David Wilson, Lorraine P. Ketzler, John Slapcinsky and Nathaniel M. Stewart ..................................... 15 Predation on Italian Newt larva, Lissotriton italicus (Amphibia, Caudata, Salamandridae), by Agabus bipustulatus (Insecta, Coleoptera, Dytiscidae) Luigi Corsetti and Gianluca Nardi........................................ 18 Behaviour, Time Management, and Foraging Modes of a West Indian Racer, Alsophis sibonius Lauren A. White, Peter J. Muelleman, Robert W. Henderson and Robert Powell . 20 Communal egg-laying and nest-sites of the Goo-Eater, Sibynomorphus mikanii (Colubridae, Dipsadinae) in southeastern Brazil Henrique B. P. Braz, Francisco L. Franco -

Predation of Oscaecilia Bassleri (Gymnophiona: Caecilidae) by Anilius Scytale (Serpentes: Aniliidae) in Southeast Peru
Nota Cuad. herpetol. 30 (1): 29-30 (2016) Predation of Oscaecilia bassleri (Gymnophiona: Caecilidae) by Anilius scytale (Serpentes: Aniliidae) in southeast Peru Jaime Villacampa 1, Andrew Whitworth1, 2 1 The Crees Foundation, Urbanización Mariscal Gamarra B-5 Zona 1 2da Etapa, Cusco, Peru. 2 Institute of Biodiversity, Animal Health and Comparative Medicine, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, G12 8QQ, UK. Recibida: 15 Abril 2015 ABSTRACT Revisada: 13 Octubre 2015 We report an event of predation between two fossorial species; the snake Anilius scytale on Aceptada: 21 Marzo 2016 the caecilian Oscaecilia bassleri, from the Manu Biosphere Reserve, southeast Peru. This is the Editor Asociado: A. Prudente first ever report of predation on O. bassleri and complements information known about the feeding ecology of A. scytale. Tropical fossorial herpetofauna species are rarely volunteer activities. The specimen was crossing one found due to their secretive lifestyles and therefore, of the pathways within the station, and was caught there is a paucity of information about their ecology and temporarily withheld in the project work area (Maritz and Alexander, 2009; Böhm et al., 2013), to be measured and photographed. At 21:30, during including feeding habits (Maschio et al., 2010). Here the measurements, the individual started to open we report upon a predation event involving two and close its mouth and began to regurgitate an fossorial species; the caecilian, Oscaecilia bassleri individual of O. bassleri (Fig. 1). (Dunn, 1942), predated by the coral pipe snake, The individual of A. scytale was 68.5 cm in Anilius scytale (Linnaeus, 1758). -

Predation by the Spider Tidarren Sisyphoides (Walckenaer, 1841) on the Ringneck Coffee Snake Ninia Diademata Baird & Girard, 1853 in Veracruz, Mexico
Herpetology Notes, volume 14: 301-302 (2021) (published online on 09 February 2021) Predation by the spider Tidarren sisyphoides (Walckenaer, 1841) on the Ringneck Coffee Snake Ninia diademata Baird & Girard, 1853 in Veracruz, Mexico Angel Ivan Contreras Calvario1, Manuel de Luna2,*, Abigail Mora Reyes1, and Xavier Contreras Calvario3 The Ringneck Coffee Snake Ninia diademata Baird & from the spider’s web, the snake spasmed and appeared Girard, 1853 is a very small, fossorial dipsadid (up to somewhat rigid. It was collected while still alive but 42 cm in total length). It has a solid black dorsum with died overnight. Both snake and spider were deposited a pale dorsal collar in the neck area and 83–106 and 73– in the herpetological and arachnological collections, 98 subcaudal scales in males and females, respectively. respectively, of the Universidad Autónoma de Nuevo These characteristics differentiate it from its only other León (voucher numbers UANL 8449 for the snake and Mexican congener, the Redback Coffee Snake N. sebae FCB-ATHER 144 for the spider). (Duméril et al., 1854), which has a red dorsum with a The spider was later identified as a female Tidarren black collar in the neck area, often possesses incomplete sisyphoides (Walckenaer, 1841) (Araneae: Theridiidae) black bands on its dorsum, and has fewer subcaudal (Fig. 1B, C) using Levi’s (1970) keys. There are various scales (41–71 in males, 37–60 in females; Heimes, 2016). recorded instances where theridiid spiders have taken Ninia diademata occurs in eastern as well as southern vertebrate prey many times larger than themselves, Mexico, excluding the Yucatán Peninsula, as well as the including snakes (Beaman and Tucker, 2014; Davis et Central American countries of Belize, Guatemala, and al., 2017; Rocha et al., 2017; Nyffeler and Vetter, 2018) Honduras. -

Different Environmental Gradients Affect Different Measures of Snake Β-Diversity in the Amazon Rainforests
Different environmental gradients affect different measures of snake β-diversity in the Amazon rainforests Rafael de Fraga1,2,3, Miquéias Ferrão1, Adam J. Stow2, William E. Magnusson4 and Albertina P. Lima4 1 Programa de Pós-Graduação em Ecologia, Instituto Nacional de Pesquisas da Amazônia, Manaus, Amazonas, Brazil 2 Department of Biological Sciences, Macquarie University, Sydney, NSW, Australia 3 Programa de Pós-graduação em Sociedade, Natureza e Desenvolvimento, Universidade Federal do Oeste do Pará, Santarém, Pará, Brazil 4 Coordenação de Biodiversidade, Instituto Nacional de Pesquisas da Amazônia, Manaus, Amazonas, Brazil ABSTRACT Mechanisms generating and maintaining biodiversity at regional scales may be evaluated by quantifying β-diversity along environmental gradients. Differences in assemblages result in biotic complementarities and redundancies among sites, which may be quantified through multi-dimensional approaches incorporating taxonomic β-diversity (TBD), functional β-diversity (FBD) and phylogenetic β-diversity (PBD). Here we test the hypothesis that snake TBD, FBD and PBD are influenced by environmental gradients, independently of geographic distance. The gradients tested are expected to affect snake assemblages indirectly, such as clay content in the soil determining primary production and height above the nearest drainage determining prey availability, or directly, such as percentage of tree cover determining availability of resting and nesting sites, and climate (temperature and precipitation) causing physiological filtering. We sampled snakes in 21 sampling plots, each covering five km2, distributed over 880 km in the central-southern Amazon Basin. We used dissimilarities between sampling sites to quantify TBD, FBD and PBD, Submitted 30 April 2018 which were response variables in multiple-linear-regression and redundancy analysis Accepted 23 August 2018 Published 24 September 2018 models. -

Check List the Journal Of
12 1 1838 the journal of biodiversity data 6 February 2016 Check List NOTES ON GEOGRAPHIC DISTRIBUTION Check List 12(1): 1838, 6 February 2016 doi: http://dx.doi.org/10.15560/12.1.1838 ISSN 1809-127X © 2016 Check List and Authors Leptophis ahaetulla (Linnaeus, 1758) (Serpentes, Colubridae): first record for the state of Rio Grande do Sul, Brazil Roberto Baptista de Oliveira1*, Christian Beier2, Giancarlo Ribeiro Bilo3, Tiago Gomes dos Santos3 and Gláucia Maria Funk Pontes4 1 Fundação Zoobotânica do Rio Grande do Sul, Museu de Ciências Naturais, Seção de Zoologia de Vertebrados, Rua Dr. Salvador França 1427, CEP 90690-000, Porto Alegre, RS, Brazil 2 Pontifícia Universidade Católica do Rio Grande do Sul, Museu de Ciências e Tecnologia, Programa de Pós-Graduação em Zoologia, Laboratório de Ornitologia, Av. Ipiranga 6681, CEP 90619-900, Porto Alegre, RS, Brazil 3 Universidade Federal do Pampa, Laboratório de Estudos em Biodiversidade Pampiana (LEBIP), Av. Antônio Trilha 1847, CEP 97300- 000, São Gabriel, RS, Brazil 4 Pontifícia Universidade Católica do Rio Grande do Sul, Museu de Ciências e Tecnologia, Setor de Herpetologia, Av. Ipiranga 6681, CEP 90619-900, Porto Alegre, RS, Brazil * Corresponding author: E-mail: [email protected] Abstract: We present the first record of Leptophis the extreme North to the state of Paraná, occupying a ahaetulla for the State of Rio Grande do Sul, Brazil. wide range of biomes, including the Amazon, Pantanal, Between November and December 2014, and February Cerrado, Caatinga and Atlantic Forest (Cunha and 2015, three specimens were found, respectively: one male Nascimento 1978; Vanzolini et al. -

First Record of Predation on the Bat Carollia Perspicillata by the False Coral Snake Oxyrhopus Petolarius in the Atlantic Rainforest
Biotemas, 25 (4), 307-309, dezembro de 2012 doi: 10.5007/2175-7925.2012v25n4p307307 ISSNe 2175-7925 Short Communication First record of predation on the bat Carollia perspicillata by the false coral snake Oxyrhopus petolarius in the Atlantic Rainforest Frederico Gustavo Rodrigues França * Rafaella Amorim de Lima Universidade Federal da Paraíba, Centro de Ciências Aplicadas e Educação Departamento de Engenharia e Meio Ambiente, CEP 58297-000, Rio Tinto – PB, Brasil * Corresponding author [email protected] Submetido em 23/03/2012 Aceito para publicação em 28/06/2012 Resumo Primeiro registro de predação do morcego Carollia perspicillata pela falsa coral Oxyrhopus petolarius na Mata Atlântica. Registros de morcegos como presas de serpentes são bastante escassos na literatura, mas trabalhos recentes têm evidenciado que essa predação não parece ser um fenômeno incomum. Apresentamos aqui o primeiro registro de predação do morcego Carollia perspicillata pela falsa coral Oxyrhopus petolarius em uma área de Mata Atlântica na região Nordeste do Brasil. Palavras-chave: Dieta; Falsa coral; Morcegos; Serpentes Abstract Records of bats as prey of snakes are very few in the literature, but recent studies have shown that this predation doesn’t seem to be an unusual phenomenon. We present here the first record of predation on the bat Carollia perspicillata by the false coral snake Oxyrhopus petolarius in an Atlantic Rainforest area in the Northeastern Brazil. Key words: Bats; Diet; False coral snake; Snakes Although bats are not considered an important and E. subflavus) (HARDY, 1957; RODRÍGUEZ; component of the diet of most snake species, snake REAGAN, 1984; KOENIG; SCHWARTZ, 2003) and predation on bats does not seem to be an unusual South American Corallus hortulanus (HENDERSON, phenomenon (SCHÄTTI, 1984; HASTINGS, 2010). -

Bibliography and Scientific Name Index to Amphibians
lb BIBLIOGRAPHY AND SCIENTIFIC NAME INDEX TO AMPHIBIANS AND REPTILES IN THE PUBLICATIONS OF THE BIOLOGICAL SOCIETY OF WASHINGTON BULLETIN 1-8, 1918-1988 AND PROCEEDINGS 1-100, 1882-1987 fi pp ERNEST A. LINER Houma, Louisiana SMITHSONIAN HERPETOLOGICAL INFORMATION SERVICE NO. 92 1992 SMITHSONIAN HERPETOLOGICAL INFORMATION SERVICE The SHIS series publishes and distributes translations, bibliographies, indices, and similar items judged useful to individuals interested in the biology of amphibians and reptiles, but unlikely to be published in the normal technical journals. Single copies are distributed free to interested individuals. Libraries, herpetological associations, and research laboratories are invited to exchange their publications with the Division of Amphibians and Reptiles. We wish to encourage individuals to share their bibliographies, translations, etc. with other herpetologists through the SHIS series. If you have such items please contact George Zug for instructions on preparation and submission. Contributors receive 50 free copies. Please address all requests for copies and inquiries to George Zug, Division of Amphibians and Reptiles, National Museum of Natural History, Smithsonian Institution, Washington DC 20560 USA. Please include a self-addressed mailing label with requests. INTRODUCTION The present alphabetical listing by author (s) covers all papers bearing on herpetology that have appeared in Volume 1-100, 1882-1987, of the Proceedings of the Biological Society of Washington and the four numbers of the Bulletin series concerning reference to amphibians and reptiles. From Volume 1 through 82 (in part) , the articles were issued as separates with only the volume number, page numbers and year printed on each. Articles in Volume 82 (in part) through 89 were issued with volume number, article number, page numbers and year. -

THE ORIGIN and EVOLUTION of SNAKE EYES Dissertation
CONQUERING THE COLD SHUDDER: THE ORIGIN AND EVOLUTION OF SNAKE EYES Dissertation Presented in Partial Fulfillment for the Requirements for the Degree of Doctor of Philosophy in the Graduate School of The Ohio State University By Christopher L. Caprette, B.S., M.S. **** The Ohio State University 2005 Dissertation Committee: Thomas E. Hetherington, Advisor Approved by Jerry F. Downhower David L. Stetson Advisor The graduate program in Evolution, John W. Wenzel Ecology, and Organismal Biology ABSTRACT I investigated the ecological origin and diversity of snakes by examining one complex structure, the eye. First, using light and transmission electron microscopy, I contrasted the anatomy of the eyes of diurnal northern pine snakes and nocturnal brown treesnakes. While brown treesnakes have eyes of similar size for their snout-vent length as northern pine snakes, their lenses are an average of 27% larger (Mann-Whitney U test, p = 0.042). Based upon the differences in the size and position of the lens relative to the retina in these two species, I estimate that the image projected will be smaller and brighter for brown treesnakes. Northern pine snakes have a simplex, all-cone retina, in keeping with a primarily diurnal animal, while brown treesnake retinas have mostly rods with a few, scattered cones. I found microdroplets in the cone ellipsoids of northern pine snakes. In pine snakes, these droplets act as light guides. I also found microdroplets in brown treesnake rods, although these were less densely distributed and their function is unknown. Based upon the density of photoreceptors and neural layers in their retinas, and the predicted image size, brown treesnakes probably have the same visual acuity under nocturnal conditions that northern pine snakes experience under diurnal conditions. -

Crocodylus Moreletii
ANFIBIOS Y REPTILES: DIVERSIDAD E HISTORIA NATURAL VOLUMEN 03 NÚMERO 02 NOVIEMBRE 2020 ISSN: 2594-2158 Es un publicación de la CONSEJO DIRECTIVO 2019-2021 COMITÉ EDITORIAL Presidente Editor-en-Jefe Dr. Hibraim Adán Pérez Mendoza Dra. Leticia M. Ochoa Ochoa Universidad Nacional Autónoma de México Senior Editors Vicepresidente Dr. Marcio Martins (Artigos em português) Dr. Óscar A. Flores Villela Dr. Sean M. Rovito (English papers) Universidad Nacional Autónoma de México Editores asociados Secretario Dr. Uri Omar García Vázquez Dra. Ana Bertha Gatica Colima Dr. Armando H. Escobedo-Galván Universidad Autónoma de Ciudad Juárez Dr. Oscar A. Flores Villela Dra. Irene Goyenechea Mayer Goyenechea Tesorero Dr. Rafael Lara Rezéndiz Dra. Anny Peralta García Dr. Norberto Martínez Méndez Conservación de Fauna del Noroeste Dra. Nancy R. Mejía Domínguez Dr. Jorge E. Morales Mavil Vocal Norte Dr. Hibraim A. Pérez Mendoza Dr. Juan Miguel Borja Jiménez Dr. Jacobo Reyes Velasco Universidad Juárez del Estado de Durango Dr. César A. Ríos Muñoz Dr. Marco A. Suárez Atilano Vocal Centro Dra. Ireri Suazo Ortuño M. en C. Ricardo Figueroa Huitrón Dr. Julián Velasco Vinasco Universidad Nacional Autónoma de México M. en C. Marco Antonio López Luna Dr. Adrián García Rodríguez Vocal Sur M. en C. Marco Antonio López Luna Universidad Juárez Autónoma de Tabasco English style corrector PhD candidate Brett Butler Diseño editorial Lic. Andrea Vargas Fernández M. en A. Rafael de Villa Magallón http://herpetologia.fciencias.unam.mx/index.php/revista NOTAS CIENTÍFICAS SKIN TEXTURE CHANGE IN DIASPORUS HYLAEFORMIS (ANURA: ELEUTHERODACTYLIDAE) ..................... 95 CONTENIDO Juan G. Abarca-Alvarado NOTES OF DIET IN HIGHLAND SNAKES RHADINAEA EDITORIAL CALLIGASTER AND RHADINELLA GODMANI (SQUAMATA:DIPSADIDAE) FROM COSTA RICA ..... -

Observations on the Behavioral Ecology of Three Species of Imantodes (Reptilia, Serpentes, Colubridae) Author(S): Robert W
Society for the Study of Amphibians and Reptiles Observations on the Behavioral Ecology of Three Species of Imantodes (Reptilia, Serpentes, Colubridae) Author(s): Robert W. Henderson and Max A. Nickerson Source: Journal of Herpetology, Vol. 10, No. 3 (Jul. 26, 1976), pp. 205-210 Published by: Society for the Study of Amphibians and Reptiles Stable URL: http://www.jstor.org/stable/1562981 . Accessed: 27/08/2013 10:35 Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp . JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Society for the Study of Amphibians and Reptiles is collaborating with JSTOR to digitize, preserve and extend access to Journal of Herpetology. http://www.jstor.org This content downloaded from 128.227.244.12 on Tue, 27 Aug 2013 10:35:27 AM All use subject to JSTOR Terms and Conditions 1976 JOURNAL OF HERPETOLOGY 10(3):205-210 Observations on the Behavioral Ecology of Three Species of Imantodes (Reptilia, Serpentes, Colubridae) Robert W. Henderson and Max A. Nickerson Vertebrate Division, Milwaukee Public Museum Milwaukee, Wisconsin 53233, USA ABSTRACT-Aspects of the behavioral ecology of Imantodes cenchoa, I. gemmistratus and /. lentiferus were studied in large glass enclosures and in a greenhouse. Imantodes is crepuscular and nocturnal and 90% of all daylight hours were spent coiled in bromeliads.