DPP-1 Quantum Numbers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lecture #2: August 25, 2020 Goal Is to Define Electrons in Atoms
Lecture #2: August 25, 2020 Goal is to define electrons in atoms • Bohr Atom and Principal Energy Levels from “orbits”; Balance of electrostatic attraction and centripetal force: classical mechanics • Inability to account for emission lines => particle/wave description of atom and application of wave mechanics • Solutions of Schrodinger’s equation, Hψ = Eψ Required boundaries => quantum numbers (and the Pauli Exclusion Principle) • Electron configurations. C: 1s2 2s2 2p2 or [He]2s2 2p2 Na: 1s2 2s2 2p6 3s1 or [Ne] 3s1 => Na+: [Ne] Cl: 1s2 2s2 2p6 3s23p5 or [Ne]3s23p5 => Cl-: [Ne]3s23p6 or [Ar] What you already know: Quantum Numbers: n, l, ml , ms n is the principal quantum number, indicates the size of the orbital, has all positive integer values of 1 to ∞(infinity) (Bohr’s discrete orbits) l (angular momentum) orbital 0s l is the angular momentum quantum number, 1p represents the shape of the orbital, has integer values of (n – 1) to 0 2d 3f ml is the magnetic quantum number, represents the spatial direction of the orbital, can have integer values of -l to 0 to l Other terms: electron configuration, noble gas configuration, valence shell ms is the spin quantum number, has little physical meaning, can have values of either +1/2 or -1/2 Pauli Exclusion principle: no two electrons can have all four of the same quantum numbers in the same atom (Every electron has a unique set.) Hund’s Rule: when electrons are placed in a set of degenerate orbitals, the ground state has as many electrons as possible in different orbitals, and with parallel spin. -

Principal, Azimuthal and Magnetic Quantum Numbers and the Magnitude of Their Values
268 A Textbook of Physical Chemistry – Volume I Principal, Azimuthal and Magnetic Quantum Numbers and the Magnitude of Their Values The Schrodinger wave equation for hydrogen and hydrogen-like species in the polar coordinates can be written as: 1 휕 휕휓 1 휕 휕휓 1 휕2휓 8휋2휇 푍푒2 (406) [ (푟2 ) + (푆푖푛휃 ) + ] + (퐸 + ) 휓 = 0 푟2 휕푟 휕푟 푆푖푛휃 휕휃 휕휃 푆푖푛2휃 휕휙2 ℎ2 푟 After separating the variables present in the equation given above, the solution of the differential equation was found to be 휓푛,푙,푚(푟, 휃, 휙) = 푅푛,푙. 훩푙,푚. 훷푚 (407) 2푍푟 푘 (408) 3 푙 푘=푛−푙−1 (−1)푘+1[(푛 + 푙)!]2 ( ) 2푍 (푛 − 푙 − 1)! 푍푟 2푍푟 푛푎 √ 0 = ( ) [ 3] . exp (− ) . ( ) . ∑ 푛푎0 2푛{(푛 + 푙)!} 푛푎0 푛푎0 (푛 − 푙 − 1 − 푘)! (2푙 + 1 + 푘)! 푘! 푘=0 (2푙 + 1)(푙 − 푚)! 1 × √ . 푃푚(퐶표푠 휃) × √ 푒푖푚휙 2(푙 + 푚)! 푙 2휋 It is obvious that the solution of equation (406) contains three discrete (n, l, m) and three continuous (r, θ, ϕ) variables. In order to be a well-behaved function, there are some conditions over the values of discrete variables that must be followed i.e. boundary conditions. Therefore, we can conclude that principal (n), azimuthal (l) and magnetic (m) quantum numbers are obtained as a solution of the Schrodinger wave equation for hydrogen atom; and these quantum numbers are used to define various quantum mechanical states. In this section, we will discuss the properties and significance of all these three quantum numbers one by one. Principal Quantum Number The principal quantum number is denoted by the symbol n; and can have value 1, 2, 3, 4, 5…..∞. -

Vibrational Quantum Number
Fundamentals in Biophotonics Quantum nature of atoms, molecules – matter Aleksandra Radenovic [email protected] EPFL – Ecole Polytechnique Federale de Lausanne Bioengineering Institute IBI 26. 03. 2018. Quantum numbers •The four quantum numbers-are discrete sets of integers or half- integers. –n: Principal quantum number-The first describes the electron shell, or energy level, of an atom –ℓ : Orbital angular momentum quantum number-as the angular quantum number or orbital quantum number) describes the subshell, and gives the magnitude of the orbital angular momentum through the relation Ll2 ( 1) –mℓ:Magnetic (azimuthal) quantum number (refers, to the direction of the angular momentum vector. The magnetic quantum number m does not affect the electron's energy, but it does affect the probability cloud)- magnetic quantum number determines the energy shift of an atomic orbital due to an external magnetic field-Zeeman effect -s spin- intrinsic angular momentum Spin "up" and "down" allows two electrons for each set of spatial quantum numbers. The restrictions for the quantum numbers: – n = 1, 2, 3, 4, . – ℓ = 0, 1, 2, 3, . , n − 1 – mℓ = − ℓ, − ℓ + 1, . , 0, 1, . , ℓ − 1, ℓ – –Equivalently: n > 0 The energy levels are: ℓ < n |m | ≤ ℓ ℓ E E 0 n n2 Stern-Gerlach experiment If the particles were classical spinning objects, one would expect the distribution of their spin angular momentum vectors to be random and continuous. Each particle would be deflected by a different amount, producing some density distribution on the detector screen. Instead, the particles passing through the Stern–Gerlach apparatus are deflected either up or down by a specific amount. -

The Quantum Mechanical Model of the Atom
The Quantum Mechanical Model of the Atom Quantum Numbers In order to describe the probable location of electrons, they are assigned four numbers called quantum numbers. The quantum numbers of an electron are kind of like the electron’s “address”. No two electrons can be described by the exact same four quantum numbers. This is called The Pauli Exclusion Principle. • Principle quantum number: The principle quantum number describes which orbit the electron is in and therefore how much energy the electron has. - it is symbolized by the letter n. - positive whole numbers are assigned (not including 0): n=1, n=2, n=3 , etc - the higher the number, the further the orbit from the nucleus - the higher the number, the more energy the electron has (this is sort of like Bohr’s energy levels) - the orbits (energy levels) are also called shells • Angular momentum (azimuthal) quantum number: The azimuthal quantum number describes the sublevels (subshells) that occur in each of the levels (shells) described above. - it is symbolized by the letter l - positive whole number values including 0 are assigned: l = 0, l = 1, l = 2, etc. - each number represents the shape of a subshell: l = 0, represents an s subshell l = 1, represents a p subshell l = 2, represents a d subshell l = 3, represents an f subshell - the higher the number, the more complex the shape of the subshell. The picture below shows the shape of the s and p subshells: (notice the electron clouds) • Magnetic quantum number: All of the subshells described above (except s) have more than one orientation. -

Magnetic Quantum Number: Describes the Orbital of the Subshell Ms Or S - Spin Quantum Number: Describes the Spin QUANTUM NUMBER VALUES
ST. LAWRENCE HIGH SCHOOL A JESUIT CHRISTIAN MINORITY INSTITUTION STUDY MATERIAL FOR CHEMISTRY (CLASS-11) TOPIC- STRUCTURE OF ATOM SUBTOPIC- QUANTUM NUMBERS PREPARED BY: MR. ARNAB PAUL CHOWDHURY SET NUMBER-03 DATE: 07.07.2020 ------------------------------------------------------------------------------------------------------------------------------- In chemistry and quantum physics, quantum numbers describe values of conserved quantities in the dynamics of a quantum system. In the case of electrons, the quantum numbers can be defined as "the sets of numerical values which give acceptable solutions to the Schrödinger wave equation for the hydrogen atom". How many quantum numbers exist? A quantum number is a value that is used when describing the energy levels available to atoms and molecules. An electron in an atom or ion has four quantum numbers to describe its state and yield solutions to the Schrödinger wave equation for the hydrogen atom. There are four quantum numbers: n - principal quantum number: describes the energy level ℓ - azimuthal or angular momentum quantum number: describes the subshell mℓ or m - magnetic quantum number: describes the orbital of the subshell ms or s - spin quantum number: describes the spin QUANTUM NUMBER VALUES According to the Pauli Exclusion Principle, no two electrons in an atom can have the same set of quantum numbers. Each quantum number is represented by either a half-integer or integer value. The principal quantum number is an integer that is the number of the electron's shell. The value is 1 or higher (never 0 or negative). The angular momentum quantum number is an integer that is the value of the electron's orbital (for example, s=0, p=1). -

Quantum Spin Systems and Their Local and Long-Time Properties Carolee Wheeler Faculty Advisor: Robert Sims
URA Project Proposal Quantum Spin Systems and Their Local and Long-Time Properties Carolee Wheeler Faculty Advisor: Robert Sims In quantum mechanics, spin is an important concept having to do with atomic nuclei, hadrons, and elementary particles. Spin may be thought of as a measure of a particle’s rotation about its axis. However, spin differs from orbital angular momentum in the sense that the particles may carry integer or half-integer quantum numbers, i.e. 0, 1/2, 1, 3/2, 2, etc., whereas orbital angular momentum may only take integer quantum numbers. Furthermore, the spin of a charged elementary particle is related to a magnetic dipole moment. All quantum mechanic particles have an inherent spin. This is due to the fact that elementary particles (such as photons, electrons, or quarks) cannot be divided into smaller entities. In other words, they cannot be viewed as particles that are made up of individual, smaller particles that rotate around a common center. Thus, the spin that elementary particles carry is an intrinsic property [1]. An important characteristic of spin in quantum mechanics is that it is quantized. The magnitude of spin takes values S = h s(s + )1 , with h being the reduced Planck’s constant and s being the spin quantum number (a non- negative integer or half-integer). Spin may also be viewed in composite particles, and is calculated by summing the spins of the constituent particles. In the case of atoms and molecules, spin is the sum of the spins of unpaired electrons [1]. Particles with spin can possess a magnetic moment. -

The Principal Quantum Number the Azimuthal Quantum Number The
To completely describe an electron in an atom, four quantum numbers are needed: energy (n), angular momentum (ℓ), magnetic moment (mℓ), and spin (ms). The Principal Quantum Number This quantum number describes the electron shell or energy level of an atom. The value of n ranges from 1 to the shell containing the outermost electron of that atom. For example, in caesium (Cs), the outermost valence electron is in the shell with energy level 6, so an electron incaesium can have an n value from 1 to 6. For particles in a time-independent potential, as per the Schrödinger equation, it also labels the nth eigen value of Hamiltonian (H). This number has a dependence only on the distance between the electron and the nucleus (i.e. the radial coordinate r). The average distance increases with n, thus quantum states with different principal quantum numbers are said to belong to different shells. The Azimuthal Quantum Number The angular or orbital quantum number, describes the sub-shell and gives the magnitude of the orbital angular momentum through the relation. ℓ = 0 is called an s orbital, ℓ = 1 a p orbital, ℓ = 2 a d orbital, and ℓ = 3 an f orbital. The value of ℓ ranges from 0 to n − 1 because the first p orbital (ℓ = 1) appears in the second electron shell (n = 2), the first d orbital (ℓ = 2) appears in the third shell (n = 3), and so on. This quantum number specifies the shape of an atomic orbital and strongly influences chemical bonds and bond angles. -

Chem 1A Chapter 7 Exercises
Chem 1A Chapter 7 Exercises Exercises #1 Characteristics of Waves 1. Consider a wave with a wavelength = 2.5 cm traveling at a speed of 15 m/s. (a) How long does it take for the wave to travel a distance of one wavelength? (b) How many wavelengths (or cycles) are completed in 1 second? (The number of waves or cycles completed per second is referred to as the wave frequency.) (c) If = 3.0 cm but the speed remains the same, how many wavelengths (or cycles) are completed in 1 second? (d) If = 1.5 cm and the speed remains the same, how many wavelengths (or cycles) are completed in 1 second? (e) What can you conclude regarding the relationship between wavelength and number of cycles traveled in one second, which is also known as frequency ()? Answer: (a) 1.7 x 10–3 s; (b) 600/s; (c) 500/s; (d) 1000/s; (e) ?) 2. Consider a red light with = 750 nm and a green light = 550 nm (a) Which light travels a greater distance in 1 second, red light or green light? Explain. (b) Which light (red or green) has the greater frequency (that is, contains more waves completed in 1 second)? Explain. (c) What are the frequencies of the red and green light, respectively? (d) If light energy is defined by the quantum formula, such that Ep = h, which light carries more energy per photon? Explain. 8 -34 (e) Assuming that the speed of light, c = 2.9979 x 10 m/s, Planck’s constant, h = 6.626 x 10 J.s., and 23 Avogadro’s number, NA = 6.022 x 10 /mol, calculate the energy: (i) per photon; (ii) per mole of photon for green and red light, respectively. -
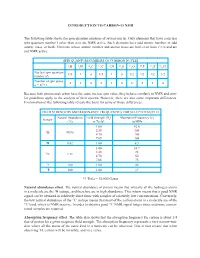
INTRODUCTION to CARBON-13 NMR the Following Table Shows The
INTRODUCTION TO CARBON-13 NMR The following table shows the spin quantum numbers of several nuclei. Only elements that have a nuclear spin quantum number I other than zero are NMR active. Such elements have odd atomic number, or odd atomic mass, or both. Elements whose atomic number and atomic mass are both even have I = 0 and are not NMR active. SPIN QUANTUM NUMBERS OF COMMON NUCLEI 1 2 12 13 14 16 17 19 31 35 1H 1H 6C 6C 7N 8O 8O 9F 15P 17Cl Nuclear spin quantum 1/2 1 0 1/2 1 0 5/2 1/2 1/2 3/2 number (I) Number of spin states 2 3 0 2 3 0 6 2 2 4 n = 2I + 1 Because both proton and carbon have the same nuclear spin value, they behave similarly in NMR and simi- lar guidelines apply to the analysis of their spectra. However, there are also some important differences. Examination of the following table reveals the basis for some of those differences. FIELD STRENGTHS AND RESONANCE FREQUENCIES FOR SELECTED NUCLEI Natural Abundance Field Strength (B ) Absorption Frequency (n) Isotope o (%) in Tesla* in MHz 1.00 42.6 2.35 100 1H 99.98 4.70 200 7.05 300 2H 0.02 1.00 6.5 1.00 10.7 2.35 25 13C 1.11 4.70 50 7.05 75 19F 100 1.00 40 31P 100 1.00 17 *1 Tesla = 10,000 Gauss Natural abundance effect. The natural abundance of proton means that virtually all the hydrogen atoms in a molecule are the 1H isotope, and therefore are in high abundance. -

The Extent of Acceptance of the Non-Separable Solution in Cylindrical Coordinates Through the Hydrogen Atom
Advanced Studies in Theoretical Physics Vol. 13, 2019, no. 8, 433 - 437 HIKARI Ltd, www.m-hikari.com https://doi.org/10.12988/astp.2019.91246 The Extent of Acceptance of the Non-separable Solution in Cylindrical Coordinates through the Hydrogen Atom Mohammad F. Alshudifat Dept. of Physics, Al al-Bayt University, Mafraq 25113, Jordan This article is distributed under the Creative Commons by-nc-nd Attribution License. Copyright c 2019 Hikari Ltd. Abstract The hydrogen atom has been used to measure the extent of accep- tance of the non-separable solution in cylindrical coordinates. This work is not representing a new solution for the hydrogen atom, but to see how the non-separable solution in cylindrical coordinates gives acceptable results when compared with the known separable solution in spherical coordinates. The current work shows that the energy states (labeled by n = 1; 2; 3; :::) were found to be consistent with the Rutherford-Bohr model. The azimuthal quantum number ` was found to have a single value for each energy state, while the magnetic quantum number m was found to have two allowed values ` and ` − 1 for each energy state, this shrank the degeneracy of the excited states (n`m) to only two different states (n``) and (n` ` − 1), this result veers from the degeneracy cal- culated using spherical coordinates. A comparison between this work and the known solution in spherical coordinates show some acceptable results especially for the energy of atomic states, which is promising to use cylindrical coordinate in quantum problems when the spherical symmetry is deformed. Keywords: Cylindrical coordinates, non-separable solution, hydrogen atom, energy states, azimuthal quantum number, angular momentum 434 Mohammad F. -

33-234 Quantum Physics Spring 2015
33-234 Quantum Physics Spring 2015 Derivation of Angular Momentum Rules in Quantum Mechanics R.M. Suter March 23, 2015 These notes follow the derivation in the text but provide some additional details and alternate explanations. Comments are welcome. For 33-234, you will not be responsible for the details of the derivations given here. We have covered the material necessary to follow the derivations so these details are presented for the ambitious and curious. All students should, however, be sure to understand the results as displayed on page 6 and following as well as the basic commutation properties given on this and the next page. Classical angular momentum. The classical angular momentum of a point particle is computed as L = R p, (1) × where R is the position, p is the linear momentum. For circular motion, this reduces to 2 2πR L = Rp = Rmv = ωmR using v = T = ωR. In the absence of external torques, L is a conserved quantity; in other words, it is constant in magnitude and direction. In the presence of a torque, τ (a vector quantity), generated by a force, F, τ = R F, the equation dL × of motion is dt = τ. In a composite system, the total angular momentum is computed as a vector sum: LT = Pi Li. We would like to determine the stationary states of angular momentum and determine the “good” quantum numbers that describe these states. For a particle bound by some potential in three dimensions, we want to know the set of physical quantities that can be used to specify the stable states or eigenstates. -

The Wave Behavior of Matter the Wave Behavior of Matter
The Wave Behavior of The Wave Behavior of Matter Matter The Uncertainty Principle • Knowing that light has a particle nature, it seems • Heisenberg’s Uncertainty Principle: on the mass scale reasonable to ask if matter has a wave nature. of atomic particles, we cannot determine exactly the • Using Einstein’s and Planck’s equations, de Broglie position, direction of motion, and speed simultaneously. h showed: λ = • For electrons: we cannot determine their momentum and mv position simultaneously. • de Broglie summarized the concepts of waves and •If ∆x is the uncertainty in position and ∆mv is the particles, with noticeable effects if the objects are small. uncertainty in momentum, then h • Problem 6.33 ∆x·∆mv ≥ 4π Prentice Hall © 2003 Chapter 6 Prentice Hall © 2003 Chapter 6 The Wave Behavior of Matter The Wave Behavior of Matter • Electron microscopy • Calculating the uncertainty in various objects (Problem • Problem 6.36 6.37) Prentice Hall © 2003 Chapter 6 Prentice Hall © 2003 Chapter 6 1 Quantum Mechanics and Quantum Mechanics and Atomic Orbitals Atomic Orbitals Orbitals and Quantum Numbers • Schrödinger proposed an equation that contains both • If we solve the Schrödinger equation, we get wave wave and particle terms. functions and energies for the wave functions. • Solving the equation leads to wave functions. • We call wave functions orbitals. • The wave function gives the shape of the electronic • Schrödinger’s equation requires 3 quantum numbers: orbital (ψ). 1. Principal Quantum Number, n. This is the same as Bohr’s • The square of the wave function, gives the probability of n. As n becomes larger, the atom becomes larger and the electron is further from the nucleus.