Kidney Function
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kidney, Renal Tubule – Dilation
Kidney, Renal Tubule – Dilation Figure Legend: Figure 1 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Dilated tubules are noted as tracts running through the cortex and outer medulla. Figure 2 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is present throughout the outer stripe of the outer medulla, extending into the cortex. Figure 3 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Slight tubule dilation is associated with degeneration and necrosis. Figure 4 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is associated with chronic progressive nephropathy. Comment: Renal tubule dilation may occur anywhere along the nephron or collecting duct system. It may occur in focal areas or as tracts running along the entire length of kidney sections (Figure 1). 1 Kidney, Renal Tubule – Dilation Renal tubule dilation may occur from xenobiotic administration, secondary mechanisms, or an unknown pathogenesis (see Kidney – Nephropathy, Obstructive (Figure 2). Dilation may result from direct toxic injury to the tubule epithelium interfering with absorption and secretion (Figure 3). It may also occur secondary to renal ischemia or from prolonged diuresis related to drug administration. Secondary mechanisms of tubule dilation may result from lower urinary tract obstruction, the deposition of tubule crystals, interstitial inflammation and/or fibrosis, and chronic progressive nephropathy (Figure 4). A few dilated tubules may be regarded as normal histologic variation. Recommendation: Renal tubule dilation should be diagnosed and given a severity grade. The location of tubule dilation should be included in the diagnosis as a site modifier. -

Renal Physiology
Renal Physiology Integrated Control of Na Transport along the Nephron Lawrence G. Palmer* and Ju¨rgen Schnermann† Abstract The kidney filters vast quantities of Na at the glomerulus but excretes a very small fraction of this Na in the final urine. Although almost every nephron segment participates in the reabsorption of Na in the normal kidney, the proximal segments (from the glomerulus to the macula densa) and the distal segments (past the macula densa) play different roles. The proximal tubule and the thick ascending limb of the loop of Henle interact with the filtration apparatus to deliver Na to the distal nephron at a rather constant rate. This involves regulation of both *Department of Physiology and filtration and reabsorption through the processes of glomerulotubular balance and tubuloglomerular feedback. Biophysics, Weill- The more distal segments, including the distal convoluted tubule (DCT), connecting tubule, and collecting Cornell Medical duct, regulate Na reabsorption to match the excretion with dietary intake. The relative amounts of Na reabsorbed College, New York, in the DCT, which mainly reabsorbs NaCl, and by more downstream segments that exchange Na for K are variable, New York; and †Kidney Disease allowing the simultaneous regulation of both Na and K excretion. Branch, National Clin J Am Soc Nephrol 10: 676–687, 2015. doi: 10.2215/CJN.12391213 Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Introduction The precise adaptation of urinary Na excretion to di- Health, Bethesda, Daily Na intake in the United States averages approx- etary Na intake results from regulated processing of an Maryland imately 180 mmol (4.2 g) for men and 150 mmol (3.5 g) ultrafiltrate of circulating plasma by the renal tubular for women (1). -

Kidney Questions
Kidney Questions Maximum Mark Question Mark Awarded 1 12 2 11 3 14 4 12 5 15 6 15 7 16 8 16 9 14 10 14 11 18 Total Mark Page 1 of 44 | WJEC/CBAC 2017 pdfcrowd.com 1. Page 2 of 44 | WJEC/CBAC 2017 pdfcrowd.com Page 3 of 44 | WJEC/CBAC 2017 pdfcrowd.com 2. Roughly 60% of the mass of the body is water and despite wide variation in the quantity of water taken in each day, body water content remains incredibly stable. One hormone responsible for this homeostatic control is antidiuretic hormone (ADH). (a) Describe the mechanisms that are triggered in the mammalian body when water intake is reduced. [6] (b) The graph below shows how the plasma concentration of antidiuretic hormone changes as plasma solute concentration rises. (i) Describe the relationship shown in the graph opposite. Page 4 of 44 | WJEC/CBAC 2017 pdfcrowd.com [2] (ii) Suggest why a person only begins to feel thirsty at a plasma solute concentration of 293 AU. [2] Secretion of antidiuretic hormone is stimulated by decreases in blood pressure and volume. These are conditions sensed by stretch receptors in the heart and large arteries. Severe diarrhoea is one condition which stimulates ADH secretion. (c) Suggest another condition which might stimulate ADH secretion. [1] Page 5 of 44 | WJEC/CBAC 2017 pdfcrowd.com 3. The diagram below shows a single nephron, with its blood supply, from a kidney. (a) (i) Name A, B and C shown on the diagram above. [3] A . B . C . (ii) Use two arrows, clearly labelled, on the nephron above, to show where the following processes take place: [2] I ultrafiltration; Page 6 of 44 | WJEC/CBAC 2017 pdfcrowd.com II selective reabsorption. -

Calcium Phosphate Microcrystals in the Renal Tubular Fluid Accelerate Chronic Kidney Disease Progression
The Journal of Clinical Investigation RESEARCH ARTICLE Calcium phosphate microcrystals in the renal tubular fluid accelerate chronic kidney disease progression Kazuhiro Shiizaki,1,2 Asako Tsubouchi,3 Yutaka Miura,1 Kinya Seo,4 Takahiro Kuchimaru,5 Hirosaka Hayashi,1 Yoshitaka Iwazu,1,6,7 Marina Miura,1,6 Batpurev Battulga,8 Nobuhiko Ohno,8,9 Toru Hara,10 Rina Kunishige,3 Mamiko Masutani,11 Keita Negishi,12 Kazuomi Kario,12 Kazuhiko Kotani,7 Toshiyuki Yamada,7 Daisuke Nagata,6 Issei Komuro,13 Hiroshi Itoh,14 Hiroshi Kurosu,1 Masayuki Murata,3 and Makoto Kuro-o1 1Division of Anti-aging Medicine, Center for Molecular Medicine, Jichi Medical University, Shimotsuke, Japan. 2Yurina Medical Park, Shimotsuga, Japan. 3Graduate School of Arts and Sciences, University of Tokyo, Tokyo, Japan. 4Division of Cell and Molecular Medicine, 5Division of Cardiology and Metabolism, Center for Molecular Medicine, 6Division of Nephrology, Department of Internal Medicine, 7Department of Clinical Laboratory Medicine, and 8Division of Histology and Cell Biology, Department of Anatomy, Jichi Medical University, Shimotsuke, Japan. 9Division of Ultrastructural Research, National Institute for Physiological Sciences, Okazaki, Japan. 10Electron Microscopy Analysis Station, Research Network and Facility Service Division, National Institute for Materials Science, Tsukuba, Japan. 11Healthcare Business Unit, Nikon Corporation, Yokohama, Japan. 12Division of Cardiovascular Medicine, Department of Internal Medicine, Jichi Medical University, Shimotsuke, Japan. 13Department of Cardiovascular Medicine, Graduate School of Medicine, University of Tokyo, Tokyo, Japan. 14Division of Endocrinology, Metabolism and Nephrology, Department of Internal Medicine, Keio University School of Medicine, Tokyo, Japan. The Western pattern diet is rich not only in fat and calories but also in phosphate. -

« Glomerulogenesis and Renal Tubular Differentiation : Role of Hnf1β »
THESE DE DOCTORAT DE L’UNIVERSITE PARIS DESCARTES Ecole doctorale « Bio Sorbonne Paris Cité », ED 562 Département Développement, Génétique, Reproduction, Neurobiologie et Vieillissement (DGRNV) Spécialité : Développement Présentée pour obtenir le titre de DOCTEUR de l’Université Paris Descartes « Glomerulogenesis and renal tubular differentiation : Role of HNF1 β » Par Mlle Arianna FIORENTINO Soutenance le 13 décembre 2016 Composition du jury : Mme. Evelyne Fischer Directrice de thèse M. Marco Pontoglio Examinateur M. Jean-Jacques Boffa Rapporteur M. Yves Allory Rapporteur M Rémi Salomon Examinateur M Jean-Claude Dussaule Examinateur Equipe "Expression Génique, Développement et Maladies" (EGDM) INSERM U1016/ CNRS UMR 8104 / Université Paris-Descartes Institut Cochin, Dpt. Développement, Reproduction et Cancer 24, Rue du Faubourg Saint Jacques, 75014 Paris, France A. Fiorentino HNF1beta in kidney development “Connaître ce n'est pas démontrer, ni expliquer. C'est accéder à la vision.” (Le Petit Prince- Antoine de Saint-Exupéry) 2 A. Fiorentino HNF1beta in kidney development Aknowledgments - Remerciements – Ringraziamenti During this long adventure of the PhD, I was surrounded by many people that I will try to thank to in these pages. In first place, I would like to thank the members of the jury that have kindly accepted to evaluate my work: Jean-Jacques Boffa, Yves Allory, Jean-Claude Dussaule and Rémi Salomon. For the supervision and the precious advices, I would like to thank Evelyne Fischer and Marco Pontoglio that overviewed all my work. I thank Evelyne, my thesis director, for the scientific exchanges of ideas, for the guidance to complete my project and for her help in difficult moments. I thank Marco for the discussions, even for the heated ones, because the pressure in the environment not only helped me to work harder on science but more importantly on my character, to face the problems and solve them. -

Glomerular Filtration I DR.CHARUSHILA RUKADIKAR Assistant Professor Physiology GFR 1
Glomerular filtration I DR.CHARUSHILA RUKADIKAR Assistant Professor Physiology GFR 1. Definition 2. Normal value 3. Variation 4. Calculation (different pressures acting on glomerular membrane) 5. Factors affecting GFR 6. Regulation of GFR 7. Measurement of GFR QUESTIONS LONG QUESTION 1. GFR 2. RENIN ANGIOTENSIN SYSTEM SHORT NOTE 1. DYNAMICS OF GFR 2. FILTRATION FRACTION 3. ANGIOTENSIN II 4. FACTORS AFFECTING GLOMERULAR FILTRATION RATE 5. REGULATION OF GFR 6. RENAL CLEARANCE TEST 7. MEASUREMENT OF GFR Collecting duct epithelium P Cells – Tall, predominant, have few organelles, Na reabsorption & vasopressin stimulated water reabsorption I cells- CT and DCT, less, having more cell organelles, Acid secretion and HCO3 transport CHARACTERISTICS OF RENAL BLOOD FLOW 600-1200 ml/min (high) AV O2 difference low (1.5 mL/dL) During exercise increases 1.5 times Low basal tone, not altered in denervated / innervated kidney VO2 in kidneys is directly proportional to RBF, Na reabsorption & GFR Not homogenous flow, cortex more & medulla less Vasa recta hairpin bend like structure, hyperosmolarity inner medulla Transplanted kidney- cortical blood flow show autoregulation & medullary blood flow don’t show autoregulation, so no TGF mechanism Neurogenic vasodilation not exist 20% of resting cardiac output, while the two kidneys make < 0.5% of total body weight. Excretory function rather than its metabolic requirement. Remarkable constancy due to autoregulation. Processes concerned with urine formation. 1. Glomerular filtration, 2. Tubular reabsorption and 3. Tubular secretion. • Filtration Fluid is squeezed out of glomerular capillary bed • Reabsorption Most nutrients, water and essential ions are returned to blood of peritubular capillaries • Secretion Moves additional undesirable molecules into tubule from blood of peritubular capillaries Glomerular filtration Glomerular filtration refers to process of ultrafiltration of plasma from glomerular capillaries into the Bowman’s capsule. -

Renal Aquaporins
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Kidney International, Vol. 49 (1996), pp.1712—1717 Renal aquaporins MARK A. KNEPPER, JAMES B. WADE, JAMES TERRIS, CAROLYN A. ECELBARGER, DAVID MARPLES, BEATRICE MANDON, CHUNG-LIN CHOU, B.K. KISHORE, and SØREN NIELSEN Laborato,y of Kidney and Electrolyte Metabolism, National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, Matyland, USA; Department of Cell Biology, Institute of Anatomy, University of Aarhus, Aarhus, Denmark; and Department of Physiology, University of Maiyland College of Medicine, Baltimore, and Department of Physiology, Unifornied Services University of the Health Sciences, Bethesda, Maiyland, USA Renal aquaporins. Aquaporins (AQPs) are a newly recognized family of gate the localization and regulation of the four renal aquaporins transmembrane proteins that function as molecular water channels. At (AQP1, AQP2, AQP3 and AQP4). least four aquaporins are expressed in the kidney where they mediate Urine is concentrated as a result of the combined function of rapid water transport across water-permeable epithelia and play critical roles in urinary concentrating and diluting processes. AQP1 is constitu- the loop of Henle, which generates a high osmolality in the renal tively expressed at extremely high levels in the proximal tubule and medulla by countercurrent multiplication, and the collecting duct, descending limb of Henle's loop. AQP2, -3 and -4 are expressed predom- which, in the presence of the antidiuretic hormone vasopressin, inantly in the collecting duct system. AQP2 is the predominant water permits osmotic equilibration between the urine and the hyper- channel in the apical plasma membrane and AQP3 and -4arefound in the basolateral plasma membrane. -

Renal Effects of Atrial Natriuretic Peptide Infusion in Young and Adult ~Ats'
003 1-3998/88/2403-0333$02.00/0 PEDIATRIC RESEARCH Vol. 24, No. 3, 1988 Copyright O 1988 International Pediatric Research Foundation, Inc. Printed in U.S.A. Renal Effects of Atrial Natriuretic Peptide Infusion in Young and Adult ~ats' ROBERT L. CHEVALIER, R. ARIEL GOMEZ, ROBERT M. CAREY, MICHAEL J. PEACH, AND JOEL M. LINDEN WITH THE TECHNICAL ASSISTANCE OF CATHERINE E. JONES, NANCY V. RAGSDALE, AND BARBARA THORNHILL Departments of Pediatrics [R.L.C., A.R.G., C.E.J., B. T.], Internal Medicine [R.M.C., J.M. L., N. V.R.], Pharmacology [M.J.P.], and Physiology [J.M.L.], University of Virginia, School of Medicine, Charlottesville, Virginia 22908 ABSTRAm. The immature kidney appears to be less GFR, glomerular filtration rate responsive to atrial natriuretic peptide (ANP) than the MAP, mean arterial pressure mature kidney. It has been proposed that this difference UeC~pV,urinary cGMP excretion accounts for the limited ability of the young animal to UN,V, urinary sodium excretion excrete a sodium load. To delineate the effects of age on the renal response to exogenous ANP, Sprague-Dawley rats were anesthetized for study at 31-32 days of age, 35- 41 days of age, and adulthod. Synthetic rat A* was infused intravenously for 20 min at increasing doses rang- By comparison to the adult kidney, the immature kidney ing from 0.1 to 0.8 pg/kg/min, and mean arterial pressure, responds to volume expansion with a more limited diuresis and glomerular filtration rate, plasma ANP concentration, natriuresis (I). A number of factors have been implicated to urine flow rate, and urine sodium excretion were measured explain this phenomenon in the neonatal kidney, including a at each dose. -

Embryology of the Kidney Rizaldy Paz Scott | Yoshiro Maezawa | Jordan Kreidberg | Susan E
1 Embryology of the Kidney Rizaldy Paz Scott | Yoshiro Maezawa | Jordan Kreidberg | Susan E. Quaggin CHAPTER OUTLINE MAMMALIAN KIDNEY DEVELOPMENT, 2 MOLECULAR GENETICS OF MODEL SYSTEMS TO STUDY KIDNEY NEPHROGENESIS, 22 DEVELOPMENT, 8 GENETIC ANALYSIS OF MAMMALIAN KIDNEY DEVELOPMENT, 15 KEY POINTS • The development of the kidney relies on reciprocal signaling and inductive interactions between neighboring cells. • Epithelial cells that comprise the tubular structures of the kidney are derived from two distinct cell lineages: the ureteric epithelia lineage that branches and gives rise to collecting ducts and the nephrogenic mesenchyme lineage that undergoes mesenchyme to epithelial transition to form connecting tubules, distal tubules, the loop of Henle, proximal tubules, parietal epithelial cells, and podocytes. • Nephrogenesis and nephron endowment requires an epigenetically regulated balance between nephron progenitor self-renewal and epithelial differentiation. • The timing of incorporation of nephron progenitor cells into nascent nephrons predicts their positional identity within the highly patterned mature nephron. • Stromal cells and their derivatives coregulate ureteric branching morphogenesis, nephrogenesis, and vascular development. • Endothelial cells track the development of the ureteric epithelia and establish the renal vasculature through a combination of vasculogenic and angiogenic processes. • Collecting duct epithelia have an inherent plasticity enabling them to switch between principal and intercalated cell identities. MAMMALIAN KIDNEY DEVELOPMENT The filtration function of the kidneys is accomplished by basic units called nephrons (Fig. 1.1). Humans on average have 1 million nephrons per adult kidney but the range of ANATOMIC OVERVIEW OF THE 4 MAMMALIAN KIDNEY total nephrons is highly variable across human populations. Each mouse kidney may contain up to 12,000–16,000 nephrons The kidney is a sophisticated, highly vascularized organ that depending on the strain.5 This wide range in nephron number plays a central role in overall body homeostasis. -
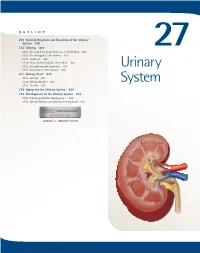
Urinary System
OUTLINE 27.1 General Structure and Functions of the Urinary System 818 27.2 Kidneys 820 27 27.2a Gross and Sectional Anatomy of the Kidney 820 27.2b Blood Supply to the Kidney 821 27.2c Nephrons 824 27.2d How Tubular Fluid Becomes Urine 828 27.2e Juxtaglomerular Apparatus 828 Urinary 27.2f Innervation of the Kidney 828 27.3 Urinary Tract 829 27.3a Ureters 829 27.3b Urinary Bladder 830 System 27.3c Urethra 833 27.4 Aging and the Urinary System 834 27.5 Development of the Urinary System 835 27.5a Kidney and Ureter Development 835 27.5b Urinary Bladder and Urethra Development 835 MODULE 13: URINARY SYSTEM mck78097_ch27_817-841.indd 817 2/25/11 2:24 PM 818 Chapter Twenty-Seven Urinary System n the course of carrying out their specific functions, the cells Besides removing waste products from the bloodstream, the uri- I of all body systems produce waste products, and these waste nary system performs many other functions, including the following: products end up in the bloodstream. In this case, the bloodstream is ■ Storage of urine. Urine is produced continuously, but analogous to a river that supplies drinking water to a nearby town. it would be quite inconvenient if we were constantly The river water may become polluted with sediment, animal waste, excreting urine. The urinary bladder is an expandable, and motorboat fuel—but the town has a water treatment plant that muscular sac that can store as much as 1 liter of urine. removes these waste products and makes the water safe to drink. -

Urine Formation in the Lamellibranchs: Evidence for Ultrafiltration and Quantitative Description
J. exp. Biol. Ill, 1-12 (1984) Wanted in Great Britain © The Company of Biologists Limited 1984 URINE FORMATION IN THE LAMELLIBRANCHS: EVIDENCE FOR ULTRAFILTRATION AND QUANTITATIVE DESCRIPTION BY F. HEVERT Institut fur Allgemeine und Spezielle Zoologie der Justus Liebig- Universitdt, D-6300 Giessen, F. R. Germany and Station de Biologie Marine, Arcachon, France Accepted 19 January 1984 SUMMARY 1. Physical and chemical parameters were measured in the Japanese oyster Crassostrea gigas to investigate whether the first step of urine formation in the lamellibranchs could be an ultrafiltration and to give a quantitative description. 2. The effective filtration pressure was not constant, but a function of time, oscillating between 31-7mmH2O and —3-8mmHzO. During the filtration, a separation of proteins took place: the protein concentration in the haemolymph was 17/imoll~l and the average molecular weight was 141000. In the filtrate, the protein concentration was Z/umoll"1 and the average molecular weight was 45 000. The marker substance inulin, applied via the gills, appeared successively within the haemolymph and the pericar- dial fluid. These findings establish the idea that the pericardial fluid is formed by ultrafiltration from the haemolymph. 3. The rate of filtration was found to be 0-4/ilg"1 min~' by quantitative analysis of the transport of the inulin. The coefficient of filtration was 4-5xlO~6mls~1cm-2mmHg-1. INTRODUCTION Among the molluscs, both cephalopods and gastropods are believed to filter a primary urine through the heart wall either into the pericardial cavity or directly into the kidney as in terrestrial pulmonate gastropods (Picken, 1937; Martin, Stewart & Harrison, 1965; Andrews & Little, 1971; Potts, 1975; Schipp & Hevert, 1981). -

Kaplan USMLE Step 1 Prep: Distribution Ion Channel Protein in Kidney
Kaplan USMLE Step 1 prep: Distribution ion channel protein in kidney FEB 3, 2020 Staff News Writer If you’re preparing for the United States Medical Licensing Examination® (USMLE®) Step 1 exam, you might want to know which questions are most often missed by test-prep takers. Check out this example from Kaplan Medical, and read an expert explanation of the answer. Also check out all posts in this series. This month’s stumper An investigator is examining the distribution of an ion channel protein in the kidney. Slices of kidney tissue are incubated in a dilute solution of a specific antibody directed against the protein. The immunoperoxidase method is then used to localize the ion channel proteins. In one area, the investigator notes epithelial cells with a brush border that are positive for the ion channel protein. Which of the following areas is most likely to show these microscopic characteristics? A. Collecting duct. B. Descending thin limb of the loop of Henle. C. Distal convoluted tubule. D. Glomerulus. E. Proximal convoluted tubule. URL: https://www.ama-assn.org/residents-students/usmle/kaplan-usmle-step-1-prep-distribution-ion-channel-protein- kidney Copyright 1995 - 2021 American Medical Association. All rights reserved. The correct answer is E. Kaplan Medical explains why The proximal convoluted tubule (PCT) is the only portion of the renal tubule in which the epithelial cells have a "brush border." The brush border is composed of microvilli, which greatly increases apical membrane surface area and thereby enhances epithelial reabsorptive capacity. The PCT recovers almost 100% of filtered organic solutes (e.g., glucose, amino acids, proteins) and about 67% of electrolytes and water, amounting to about 120 L of the daily filtered load.