Crude Oil Infiltration and Movement in First-Year Sea Ice: Impacts on Ice-Associated Biota and Physical Constraints
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Numerical Modelling of Snow and Ice Thicknesses in Lake Vanajavesi, Finland
View metadata, citation and similar papers at core.ac.uk SERIES A brought to you by CORE DYNAMIC METEOROLOGY provided by Helsingin yliopiston digitaalinen arkisto AND OCEANOGRAPHY PUBLISHED BY THE INTERNATIONAL METEOROLOGICAL INSTITUTE IN STOCKHOLM Numerical modelling of snow and ice thicknesses in Lake Vanajavesi, Finland By YU YANG1,2*, MATTI LEPPA¨ RANTA2 ,BINCHENG3,1 and ZHIJUN LI1, 1State Key Laboratory of Coastal and Offshore Engineering, Dalian University of Technology, Dalian 116024, China; 2Department of Physics, University of Helsinki, PO Box 48, FI-00014 Helsinki, Finland; 3Finnish Meteorological Institute, PO Box 503, FI-00101, Helsinki, Finland (Manuscript received 27 March 2011; in final form 7 January 2012) ABSTRACT Snow and ice thermodynamics was simulated applying a one-dimensional model for an individual ice season 2008Á2009 and for the climatological normal period 1971Á2000. Meteorological data were used as the model input. The novel model features were advanced treatment of superimposed ice and turbulent heat fluxes, coupling of snow and ice layers and snow modelled from precipitation. The simulated snow, snowÁice and ice thickness showed good agreement with observations for 2008Á2009. Modelled ice climatology was also reasonable, with 0.5 cm d1 growth in DecemberÁMarch and 2 cm d1 melting in April. Tuned heat flux from waterto ice was 0.5 W m 2. The diurnal weather cycle gave significant impact on ice thickness in spring. Ice climatology was highly sensitive to snow conditions. Surface temperature showed strong dependency on thickness of thin ice (B0.5 m), supporting the feasibility of thermal remote sensing and showing the importance of lake ice in numerical weather prediction. -

2Growth, Structure and Properties of Sea
Growth, Structure and Properties 2 of Sea Ice Chris Petrich and Hajo Eicken 2.1 Introduction The substantial reduction in summer Arctic sea ice extent observed in 2007 and 2008 and its potential ecological and geopolitical impacts generated a lot of attention by the media and the general public. The remote-sensing data documenting such recent changes in ice coverage are collected at coarse spatial scales (Chapter 6) and typically cannot resolve details fi ner than about 10 km in lateral extent. However, many of the processes that make sea ice such an important aspect of the polar oceans occur at much smaller scales, ranging from the submillimetre to the metre scale. An understanding of how large-scale behaviour of sea ice monitored by satellite relates to and depends on the processes driving ice growth and decay requires an understanding of the evolution of ice structure and properties at these fi ner scales, and is the subject of this chapter. As demonstrated by many chapters in this book, the macroscopic properties of sea ice are often of most interest in studies of the interaction between sea ice and its environment. They are defi ned as the continuum properties averaged over a specifi c volume (Representative Elementary Volume) or mass of sea ice. The macroscopic properties are determined by the microscopic structure of the ice, i.e. the distribution, size and morphology of ice crystals and inclusions. The challenge is to see both the forest, i.e. the role of sea ice in the environment, and the trees, i.e. the way in which the constituents of sea ice control key properties and processes. -

The Roland Von Glasow Air-Sea-Ice Chamber (Rvg-ASIC)
https://doi.org/10.5194/amt-2020-392 Preprint. Discussion started: 14 October 2020 c Author(s) 2020. CC BY 4.0 License. The Roland von Glasow Air-Sea-Ice Chamber (RvG-ASIC): an experimental facility for studying ocean/sea-ice/atmosphere interactions Max Thomas1, James France1,2,3, Odile Crabeck1, Benjamin Hall4, Verena Hof5, Dirk Notz5,6, Tokoloho Rampai4, Leif Riemenschneider5, Oliver Tooth1, Mathilde Tranter1, and Jan Kaiser1 1Centre for Ocean and Atmospheric Sciences, School of Environmental Sciences, University of East Anglia, UK, NR4 7TJ 2British Antarctic Survey, Natural Environment Research Council, Cambridge CB3 0ET, UK 3Department of Earth Sciences, Royal Holloway, University of London, Egham TW20 0EX, UK 4Chemical Engineering Deptartment, University of Cape Town, South Africa 5Max Planck Institute for Meteorology, Hamburg, Germany 6Center for Earth System Research and Sustainability (CEN), University of Hamburg, Germany Correspondence: Jan Kaiser ([email protected]) Abstract. Sea ice is difficult, expensive, and potentially dangerous to observe in nature. The remoteness of the Arctic and Southern Oceans complicates sampling logistics, while the heterogeneous nature of sea ice and rapidly changing environmental conditions present challenges for conducting process studies. Here, we describe the Roland von Glasow Air-Sea-Ice Chamber (RvG-ASIC), a laboratory facility designed to reproduce polar processes and overcome some of these challenges. The RvG- 5 ASIC is an open-topped 3.5 m3 glass tank housed in a coldroom (temperature range: -55 to +30 oC). The RvG-ASIC is equipped with a wide suite of instruments for ocean, sea ice, and atmospheric measurements, as well as visible and UV lighting. -

Ice Trials in Antarctica • New Rules on the Northern Sea Route • Processing Barge to the Arctic • Equipment for the Navy in This Issue
Arctic Passion News No. 1 | 2020 | issue 19 • Ice trials in Antarctica • New rules on the Northern Sea Route • Processing barge to the Arctic • Equipment for the Navy In this issue Page 4 Page 8 Page 11 Page 16 New rules on the Northern Xue Long 2 in Equipment for the Navy Barge for mining project Sea Route ice trials Table of contents From the Managing Director.................................. 3 Front cover New regime and regulations on the NSR...............4 Sami Saarinen spent six weeks travelling to Antarctica Xue Long 2 in successful ice trials.......................... 8 and back, onboard both of China’s icebreakers Xue Equipment for Navy corvettes.......................... ….11 Long and Xue Long 2. Read about his voyage and Safe and reliable shipping of crude oil................. 12 Xue Long 2’s ice trials on page 8. Feasibility study for Qilak LNG.........................….14 Aalto Ice Tank opens.............................................15 Contact details Pavlovskoe mining project.....................................16 AKER ARCTIC TECHNOLOGY INC Reducing ice friction since 1969 ...........................18 Merenkulkijankatu 6, FI-00980 HELSINKI Active Heeling systems.........................................20 Tel.: +358 10 323 6300 News in brief.........................................................21 www.akerarctic.fi Announcements....................................................23 Study tour to Gothenburg.....................................24 Join our subscription list Our services Please send your message to www.akerarctic.fi -

Chronology, Stable Isotopes, and Glaciochemistry of Perennial Ice in Strickler Cavern, Idaho, USA
Investigation of perennial ice in Strickler Cavern, Idaho, USA Chronology, stable isotopes, and glaciochemistry of perennial ice in Strickler Cavern, Idaho, USA Jeffrey S. Munroe†, Samuel S. O’Keefe, and Andrew L. Gorin Geology Department, Middlebury College, Middlebury, Vermont 05753, USA ABSTRACT INTRODUCTION in successive layers of cave ice can provide a record of past changes in atmospheric circula- Cave ice is an understudied component The past several decades have witnessed a tion (Kern et al., 2011a). Alternating intervals of of the cryosphere that offers potentially sig- massive increase in research attention focused ice accumulation and ablation provide evidence nificant paleoclimate information for mid- on the cryosphere. Work that began in Antarc- of fluctuations in winter snowfall and summer latitude locations. This study investigated tica during the first International Geophysi- temperature over time (e.g., Luetscher et al., a recently discovered cave ice deposit in cal Year in the late 1950s (e.g., Summerhayes, 2005; Stoffel et al., 2009), and changes in cave Strickler Cavern, located in the Lost River 2008), increasingly collaborative efforts to ex- ice mass balances observed through long-term Range of Idaho, United States. Field and tract long ice cores from Antarctica (e.g., Jouzel monitoring have been linked to weather patterns laboratory analyses were combined to de- et al., 2007; Petit et al., 1999) and Greenland (Schöner et al., 2011; Colucci et al., 2016). Pol- termine the origin of the ice, to limit its age, (e.g., Grootes et al., 1993), satellite-based moni- len and other botanical evidence incorporated in to measure and interpret the stable isotope toring of glaciers (e.g., Wahr et al., 2000) and the ice can provide information about changes compositions (O and H) of the ice, and to sea-ice extent (e.g., Serreze et al., 2007), field in surface environments (Feurdean et al., 2011). -

Peer Review of the Finnish Shipbuilding Industry Peer Review of the Finnish Shipbuilding Industry
PEER REVIEW OF THE FINNISH SHIPBUILDING INDUSTRY PEER REVIEW OF THE FINNISH SHIPBUILDING INDUSTRY FOREWORD This report was prepared under the Council Working Party on Shipbuilding (WP6) peer review process. The opinions expressed and the arguments employed herein do not necessarily reflect the official views of OECD member countries. The report will be made available on the WP6 website: http://www.oecd.org/sti/shipbuilding. This document and any map included herein are without prejudice to the status of or sovereignty over any territory, to the delimitation of international frontiers and boundaries and to the name of any territory, city or area. © OECD 2018; Cover photo: © Meyer Turku. You can copy, download or print OECD content for your own use, and you can include excerpts from OECD publications, databases and multimedia products in your own documents, presentations, blogs, websites and teaching materials, provided that suitable acknowledgment of OECD as source and copyright owner is given. All requests for commercial use and translation rights should be submitted to [email protected]. 2 PEER REVIEW OF THE FINNISH SHIPBUILDING INDUSTRY TABLE OF CONTENTS FOREWORD ................................................................................................................................................... 2 EXECUTIVE SUMMARY ............................................................................................................................. 4 PEER REVIEW OF THE FINNISH MARITIME INDUSTRY .................................................................... -
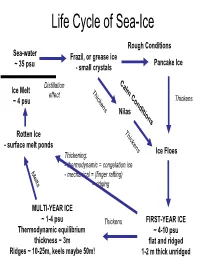
Life Cycle of Sea-Ice
Life Cycle of Sea-Ice Rough Conditions Sea-water Frazil, or grease ice Pancake Ice ~ 35 psu - small crystals C Distillation a Ice Melt T lm effect hi C ~ 4 psu ck o Thickens ens nd Nilas itio ns T hi Rotten Ice c - surface melt ponds kens Ice Floes Thickening: - thermodynamic = congelation ice M e - mechanical = (finger rafting) l ts = ridging MULTI-YEAR ICE ~ 1-4 psu Thickens FIRST-YEAR ICE Thermodynamic equilibrium ~ 4-10 psu thickness ~ 3m flat and ridged Ridges ~ 10-25m, keels maybe 50m! 1-2 m thick unridged Internal Structure of Sea Ice Brine Channels within the ice (~width of human hair) Brine rejected from ice (4-10psu), away from surface, but concentrates in brine channels long crystals as congelation ice (small volume but VERY HIGH SALINITIES) (frozen on from below) -6 deg C -10 deg C -21 deg C 100psu 145psu 216psu Pictures from AWI Brine Volume and Salinity From Thomas and Dieckmann 2002, Science .... adapted from papers by Hajo Eichen Impacts of Sea-ice on the Ocean ICE FORMATION and PRESENCE Wind - brine rejection - Ocean-Atmos momentum barrier - Ocean-Atmos heat barrier - ice edge processes (e.g. upwelling) - keel stirring (i.e. mixing, but < wind) Ocean 10psu MELTING ICE Fresh 35psu - stratification (fresher water) (cf. distillation as ice moves from formation region) Saltier - transport of sediment, etc S increases START FREEZE MELT Impacts of Sea-ice on the Atmosphere ICE PRESENCE - albedo change - Ocean-Atmos momentum barrier - Ocean-Atmos heat barrier Water Sky Sea Smoke Heat balance S=Shortwave radiation from sun (reflects off clouds and surface) albedo= how much radiation reflects from surface albedo of ice ~ 0.8 albedo of water ~ 0.04 (if sun overhead) L=Longwave radiation (from surface and clouds) F=Heat flux from Ocean M=Melt (snow and ice) From N. -

Frazil Ice Formation in the Polar Oceans
Frazil Ice Formation in the Polar Oceans Nikhil Vibhakar Radia Department of Earth Sciences, UCL A thesis submitted for the degree of Doctor of Philosophy Supervisor: D. L. Feltham August, 2013 1 I, Nikhil Vibhakar Radia, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. SIGNED 2 Abstract Areas of open ocean within the sea ice cover, known as leads and polynyas, expose ocean water directly to the cold atmosphere. In winter, these are regions of high sea ice production, and they play an important role in the mass balance of sea ice and the salt budget of the ocean. Sea ice formation is a complex process that starts with frazil ice crys- tal formation in supercooled waters, which grow and precipitate to the ocean surface to form grease ice, which eventually consolidates and turns into a layer of solid sea ice. This thesis looks at all three phases, concentrating on the first. Frazil ice comprises millimetre- sized crystals of ice that form in supercooled, turbulent water. They initially form through a process of seeding, and then grow and multiply through secondary nucleation, which is where smaller crystals break off from larger ones to create new nucleii for further growth. The increase in volume of frazil ice will continue to occur until there is no longer super- cooling in the water. The crystals eventually precipitate to the surface and pile up to form grease ice. The presence of grease ice at the ocean surface dampens the effects of waves and turbulence, which allows them to consolidate into a solid layer of ice. -

50 Years of Ice Model Testing
SHIP CONSULTING ICE MODEL OFFSHORE Design & Engineering & Project Development & Full Scale Testing Development 50 Years of Ice Model Testing Topi Leiviskä Head of Research and Testing Aker Arctic Technology inc 28.2.2019 4 March, 2019 Slide 1 Manhattan project (ESSO) ¡ Mid 1960s large oil reservuars were localized in the Alaskan Noth Slope ¡ It could be feasibly transported to the market through the Northwest Passage ¡ A decision was taken to modify an existing 106,000 DWT tanker, SS Manhattan ¡ Manhattan was refitted for the arctic voyage with an icebreaker bow in 1968–69 ¡ During the retrofit process, the oil company Esso (Humble Oil) suggested to study the performance in ice of the newly designed bow in model-scale ¡ Esso decided to invest in construction of the first ice model testing facility in Finland ¡ The first ice model test basin in Finland was ready for testing at the end of 1969 4 March, 2019 Slide 2 Icebreaker design in Finland 1933-1970 Name (previous names) Year Name (previous names) Year Louhi (ex-Sisu) 1939 Kiev 1965 Voima 1954 Askiplios (ex-Hanse) 1966 Kapitan Belousov 1954 Murmansk 1968 Kapitan Voronin 1955 Varma 1968 Kapitan Meheklov 1956 Vladivostok 1969 Oden 1957 Polar Star (ex-Njord) 1969 Karu (ex-Karhu) 1958 Dudinka (ex-Apu) 1970 Murtaja 1959 Ale 1973 Moskva 1960 Mega (ex-Aatos, Teuvo) 1973 Sampo 1961 Ermak 1974 Leningrad 1961 Atle 1974 Tor 1964 Urho 1975 4 March, 2019 Slide 3 WIMB Presentation Video 4 March, 2019 Slide 4 Time line of the ice model testing facilities (Kværner) Wärtsilä Masa-Yards Wärtsilä Arctic -

Anchor Ice and Bottom-Freezing in High-Latitude Marine Sedimentary Environments: Observations from the Alaskan Beaufort Sea
ANCHOR ICE AND BOTTOM-FREEZING IN HIGH-LATITUDE MARINE SEDIMENTARY ENVIRONMENTS: OBSERVATIONS FROM THE ALASKAN BEAUFORT SEA by Erk Reimnitz, E. W. Kempema, and P. W. Barnes U.S. Geological Survey Menlo Park, California 94025 Final Report Outer Continental Shelf Environmental Assessment Program Research Unit 205 1986 257 This report has also been published as U.S. Geological Survey Open-File Report 86-298. ACKNOWLEDGMENTS This study was funded in part by the Minerals Management Service, Department of the Interior, through interagency agreement with the National Oceanic and Atmospheric Administration, Department of Commerce, as part of the Alaska Outer Continental Shelf Environmental Assessment Program. We thank D. A. Cacchione for his thoughtful review of the manuscript. TABLE OF CONTENTS Page ACKNOWLEDGMENTS . ...259 INTRODUCTION . 263 REGIONAL SETTING . ...264 INDIRECT EVIDENCE FOR ANCHOR ICE IN THE BEAUFORT SEA . ...266 DIVER OBSERVATIONS OF ANCHOR ICE AND ICE-BONDED SEDIMENTS . ...271 DISCUSSION AND CONCLUSION . ...274 REFERENCES CITED . ...278 INTRODUCTION As early as 1705 sailors observed that rivers sometimes begin to freeze from the bottom (Barnes, 1928; Piotrovich, 1956). Anchor ice has been observed also in lakes and the sea (Zubov, 1945; Dayton, et al., 1969; Foulds and Wigle, 1977; Martin, 1981; Tsang, 1982). The growth of anchor ice implies interactions between ice and the sub- strate, and a marked change in the sedimentary environment. However, while the literature contains numerous observations that imply sediment transport, no studies have been conducted on the effects of anchor ice growth on sediment dynamics and bedforms. Underwater ice is the general term for ice formed in the supercooled water column. -

Optical Properties of the Ice Cover on Vendyurskoe Lake, Russian Karelia (1995–2012)
Annals of Glaciology 54(62) 2013 doi: 10.3189/2013AoG62A179 121 Optical properties of the ice cover on Vendyurskoe lake, Russian Karelia (1995–2012) G. ZDOROVENNOVA, R. ZDOROVENNOV, N. PALSHIN, A. TERZHEVIK Northern Water Problems Institute, Karelian Scientific Centre, Russian Academy of Sciences, Petrozavodsk, Russia E-mail: [email protected] ABSTRACT. Solar radiation penetrating the ice is one of the most important factors that determine the functioning of lake ecosystem in late winter. Parameterization of the attenuation of solar radiation in the snow-ice sheet is an essential tool in the study of the light regime of ice-covered lakes. The optical properties of the snow-ice sheet in Vendyurskoe lake, northwestern Russia, are investigated on the basis of long-term field observations (1995–2012). The four-layer approach (snow, white ice, slush and congelation ice) is used to study the attenuation of the downwelling planar irradiance in the snow-ice sheet. The bulk attenuation coefficients for four layers (18.8 m–1 for snow, 6 m–1 for white ice, 3.5 m–1 for slush and 2.1 m–1 for congelation ice) are calculated by the quasi-Newton method. A comparison of observed and calculated values of the irradiance beneath the ice shows that the determined coefficients adequately describe the attenuation of the downwelling irradiance by snow-ice cover. INTRODUCTION during intense melting have been studied insufficiently Ice and snow cover not only prevents gas exchange, but also because of the difficulty of carrying out in situ measurements insulates water bodies from the thermodynamic effect of the on weakened ice. -

Observing Alaska Lake and River Freeze-Up Through Fresh Eyes on Ice
Observing Alaska Lake and River Freeze-up through Fresh Eyes on Ice Chris Dana Laura Katie Arp Brown Oxtoby Spellman Hydrologist Geographer Ecologist & Ecologist & Educator Educator Kuskokwim River above Kwethluk 1819223141727710-NovDec-OctNovDec-2019--20192019 14-Oct-2019 FirstskimpanHalloweenNewMostlyBeringGradualAnotherComplete-ice -iceObservationice Seacovered forming refreezemeltformingaccumulating refreeze warmstorm event withand -episodeand upbringof-up! accumulatingIcejumbled snowfall another ice melt event Outline of Webinar 1. Overview of Fresh Eyes on Ice – a new freshwater ice observation network for Alaska 2. Freeze-up process: what it signals and why it matters 3. River and lake freeze-up at global to local scales 4. This year’s freeze up in context of past 5. Community and citizen science observations of freeze-up Connecting Arctic Communities through a Revitalized and Modernized Freshwater Ice Observation Network Investigators Chris Arp Dana Brown Laura Oxtoby Katie Spellman Team Members Karin Bodony Allen Bondurant Sarah Clement Melanie Engram Tohru Saito Theresa Villano Peter Webley Collaborators Alaska DNR (Parks and ADF&G) Bethel Search & Rescue NNA/AON USFWS, NPS, and BLM #1836523 River Watch Program (NOAA) (2019 – 2024) … and many Alaska communities http://fresheyesonice.org/ & schools Connecting a Landscape of Water and People through Observations Surveys & Field Studies Remote Sensing Real-time Cameras & Ice Buoys Archiving & Analysis of Historic Data Community-based Monitoring & Science Education Learning from