Official Journal L 31 of the European Union
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
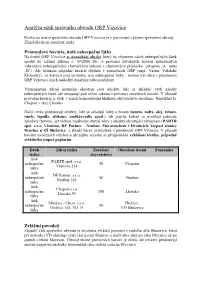
Analýza Rizik Správního Obvodu ORP Vizovice
Analýza rizik správního obvodu ORP Vizovice Riziko na území správního obvodu ORP Vizovice je v porovnání s jinými správními obvody Zlínského kraje poměrně nízké. Průmyslové havárie, únik nebezpečné látky Na území ORP Vizovice se nenachází objekt, který by objemem zásob nebezpečných látek spadal do režimu zákona č. 59/2006 Sb., o prevenci závažných havárií způsobených vybranými nebezpečnými chemickými látkami a chemickými přípravky (skupina „A“ nebo „B“). Ale účinkem případné havárie objektu v sousedících ORP (např. Vsetín, Valašské Klobouky), ve kterých jsou umístěny tyto nebezpečné látky, mohou být obce v působnosti ORP Vizovice jejich následky zasaženy nebo postiženy. Významnými zdroji možného ohrožení jsou objekty, kde se skladují větší zásoby nebezpečných látek, ale nespadají pod režim zákona o prevenci závažných havárií. V případě provozní havárie je však v jejich bezprostřední blízkosti obyvatelstvo ohroženo. Například fa. Cheport v obci Lhotsko Další rizika představují objekty, kde se skladují látky a hmoty benzin, nafta, olej, toluen, tmely, lepidla, silikony, změkčovadla apod.), při jejichž hoření se uvolňují jedovaté zplodiny (toxiny), jež mohou zasáhnout obytné zóny a objekty ohrožující výbuchem (PARTR spol. s.r.o. Všemina, DF Partner - Neubuz, Moraviachem v Hrobicích, čerpací stanice Benzina u ZŠ Slušovice a sklady barev rozmístěné v působnosti ORP Vizovice. V případě havárie uvedených objektů a dle jejího rozsahu se předpokládá vyhlášení třetího, případně zvláštního stupně poplachu. Druh Zdroj rizika Zasažené Ohrožené území Poznámka rizika obyvatelstvo únik PARTR spol. s.r.o. nebezpečné 50 Všemina Všemina 234 látky únik DF Partner, s.r.o. nebezpečné 50 Neubuz Neubuz 165 látky únik Cheport s.r.o nebezpečné 100 Lhotsko Lhotsko 93 látky únik Morávia - Chem, s.r.o. -

Commission Implementing Decision (EU) 2018/883
Status: Point in time view as at 31/10/2019. Changes to legislation: There are currently no known outstanding effects for the Commission Implementing Decision (EU) 2018/883. (See end of Document for details) Commission Implementing Decision (EU) 2018/883 of 18 June 2018 amending the Annex to Implementing Decision 2014/709/EU concerning animal health control measures relating to African swine fever in certain Member States (notified under document C(2018) 3942) (Text with EEA relevance) COMMISSION IMPLEMENTING DECISION (EU) 2018/883 of 18 June 2018 amending the Annex to Implementing Decision 2014/709/EU concerning animal health control measures relating to African swine fever in certain Member States (notified under document C(2018) 3942) (Text with EEA relevance) THE EUROPEAN COMMISSION, Having regard to the Treaty on the Functioning of the European Union, Having regard to Council Directive 89/662/EEC of 11 December 1989 concerning veterinary checks in intra-Community trade with a view to the completion of the internal market(1), and in particular Article 9(4) thereof, Having regard to Council Directive 90/425/EEC of 26 June 1990 concerning veterinary and zootechnical checks applicable in intra-Community trade in certain live animals and products with a view to the completion of the internal market(2), and in particular Article 10(4) thereof, Having regard to Council Directive 2002/99/EC of 16 December 2002 laying down the animal health rules governing the production, processing, distribution and introduction of products of animal origin for human consumption(3), and in particular Article 4(3) thereof, Whereas: (1) Commission Implementing Decision 2014/709/EU(4) lays down animal health control measures in relation to African swine fever in certain Member States, where there have been confirmed cases of that disease in domestic or feral pigs (the Member States concerned). -

Celková Požadovaná
Celková požadovaná Příjmení žadatele Obec žadatele Obec realizace výše dotace Kč Abdul Vsetín Vsetín 74802 Adamec Uherský Brod Bojkovice 127500 Adamec Nezdenice Nezdenice 107500 Adámek Rožnov pod Radhoštěm Velká Lhota 127500 Adámek Rožnov pod Radhoštěm Rožnov pod Radhoštěm 127500 Adámek Jablůnka Jablůnka 107500 Adamík Ludslavice Ludslavice 90000 Adamík Lechotice Lechotice 107500 Adámková Vsetín Vsetín 102500 Adamová Zlín Hrobice 102500 Adamuška Brumov-Bylnice Brumov-Bylnice 97500 Ambruz Březolupy Březolupy 102500 Andrlík Záhorovice Záhorovice 102500 Andrys Dolní Bečva Dolní Bečva 127500 Antošová Slavičín Hostětín 120000 Arnesen Janušová Nezdenice Nezdenice 73350 Bábek Střížovice Střížovice 127500 Babíčková Provodov Prostřední Bečva 102500 Babovec Janová Janová 102500 Baček Újezd u Valašských Klobouk Újezd 87500 Baďurová Zádveřice - Raková Zádveřice-Raková 127500 Bachan Uherský Ostroh Uherský Ostroh 127500 Bachanová Uherský Ostroh Uherský Ostroh 127500 Bajza Zlín Zlín 127500 Balaštíková Tupesy Tupesy 102500 Balcar Karolinka Karolinka 102500 Balvan Valašské Meziříčí Valašské Meziříčí 127500 Bambuch Francova Lhota Francova Lhota 90000 Bambuch Kelč Kelč 127500 Bambušek Horní Bečva Horní Bečva 127500 Barcuch Slavičín Slavičín 102500 Baroň Mikulůvka Mikulůvka 107500 Barot Zlín Zlín 102500 Bártek Ratiboř Ratiboř 107500 Bartko Uherský Brod Lopeník 127500 Bartková Nivnice Nivnice 127500 Bártková Slavkov pod Hostýnem Valašské Meziříčí 102500 Bartoníková Otrokovice Otrokovice 127500 Bartošek Kudlovice Kudlovice 102500 Bartošík Slavičín Slavičín -

Nařízení Státní Veterinární Správy
Č. j.: SVS/2014/052369-Z Nařízení Státní veterinární správy Krajská veterinární správa Státní veterinární správy pro Zlínský kraj (dále jen „KVS“), jako správní orgán příslušný podle § 47 odst. 4 a 8 a podle § 49 odst. 1 písm. c) zákona č. 166/1999 Sb., o veterinární péči a změně některých souvisejících zákonů, ve znění pozdějších předpisů (dále jen „veterinární zákon“), v souladu s § 54 odst. 2 písm. a) veterinárního zákona a s § 15 odst. 1. písm. b) a e) a s § 76 odst. 3 písm. a) veterinárního zákona nařizuje tato mimořádná veterinární opatření při výskytu a k zamezení šíření nebezpečné nákazy-moru včelího plodu v části územního obvodu Zlínského kraje. Čl. 1 Úvodní ustanovení (1) Toto nařízení je závazné pro všechny chovatele včel s chovy včel umístěných na katastrálních územích vyjmenovaných v článku 2 tohoto Nařízení, a to bez ohledu, zda jsou organizovaní v Českém svazu včelařů (dále jen „ČSV“) nebo v jiné organizaci anebo neorganizovaní. (2) Mimořádná veterinární opatření (ochranná a zdolávací opatření) se nařizují k zajištění ochrany zdravých chovů včel před nebezpečnou nákazou–morem včelího plodu a jejímu zdolání, a to vzhledem ke zjištění výskytu této nebezpečné nákazy–moru včelího plodu, potvrzeného nálezem původce této nákazy laboratorním vyšetřením a návazně vymezených ohnisek nákazy v chovech včel držených na stanovištích včel ve Zlínském kraji těmito rozhodnutími: a) Rozhodnutím Krajské veterinární správy Státní veterinární správy pro Zlínský kraj č. j. SVS/2014/051645-Z ze dne 8. 7. 2014 v katastru obce Držková, okres Zlín, s účinností od 8. 7. 2014, b) Rozhodnutím Krajské veterinární správy Státní veterinární správy pro Zlínský kraj č. -

Obec Název Zařízení Adresa Provozní Doba Kontaktní Osoba Telefon E
Obec Název zařízení Adresa Provozní doba Kontaktní osoba Telefon E-mail Webové stránky Další informace Biskupice 120, Obecní knihovna Biskupice 763 41 Biskupice u čtvrtek 16:30 - 18:30 Ilona Kadlčíková 721 775 148 [email protected] - Biskupice Luhačovic Biskupice 4, pondělí - pátek 7:00 - Biskupice Česká pošta, s.p. 763 41 Biskupice u - 577 136 043 - - 11:00, 13:00 - 15:15 Luhačovic Základní škola a Biskupice 62, Mateřská škola Mgr. Michaela Biskupice 763 41 Biskupice u - 577 136 055 - http://zsams.biskupiceuluhacovic.cz/ Biskupice, okres Zlín, Žáková Luhačovic příspěvková organizace Obecní knihovna Bohuslavice nad Vláří Bohuslavice nad Vláří pátek 16:00 - 19:00 Naděžda Urbánková 736 742 848 [email protected] http://www.bohuslavicenadvlari.knihovna.info/ Bohuslavice nad Vláří 62, 763 21 Slavičín Základní škola a Mateřská škola Bohuslavice u Zlína Mgr. Lenka 577 991 006 [email protected] Bohuslavice u Zlína Bohuslavice u Zlína, 221, 763 51 - http://www.zsms-bohuslaviceuzlina.cz Vavrušová 733 508 680 [email protected] okres Zlín, příspěvková Bohuslavice u Zlína organizace Obecní knihovna Březnice 20, 760 01 Březnice pondělí 17:00 - 19:00 Irena Haluzová 724 191 858 [email protected] http://breznice.knihovna.cz/ Březnice Zlín Základní škola a Mateřská škola Březnice 15, 760 01 Březnice - Mgr. Jana Vojtková 577 991 166 [email protected] http://zs-a-ms-breznice.webnode.cz/ Březnice, okres Zlín, Zlín příspěvková organizace pondělí, úterý, čtvrtek, pátek 8:00 - 11:00, Březůvky 1, 763 45 Březůvky Česká pošta, s.p. 13:30 - 15:15 - 577 159 830 - - Březůvky středa 8:00 - 11:00, 14:30 - 18:00 Ordinace praktického Březůvky 1, 763 45 pondělí 12:30 -14:00 Březůvky MUDr. -

Profil Města Valašské Klobouky
Profil města Valašské Klobouky Profil města Valašské Klobouky Obsah: Postavení města v rámci České republiky 4 Historie města a jeho vývoj 4 Pamětihodnosti a významní rodáci 6 Základní geografické údaje 7 Klimatické podmínky 7 Přírodní zdroje 7 ŽIVOTNÍ PROSTŘEDÍ Kvalita ovzduší 8 Kvalita vody 8 Pitná voda 10 Hlukové zatížení 11 Nakládání s odpady 11 Přírodní lokality, chráněná území 11 Veřejná zeleň 12 TECHNICKÁ INFRASTRUKTURA Zásobování vodou 13 Kanalizace a čištění odpadních vod 15 Elektrorozvody 19 Plyn 23 Tepelná energie 24 2 Veřejné osvětlení 24 Telekomunikace a radiokomunikace, internet 25 Doprava a logistické vazby 26 Dopravní obslužnost 27 Komunikace 28 LIDSKÉ ZDROJE Demografie 29 Pracovní síla 29 SOCIÁLNÍ INFRASTRUKTURA Bydlení 30 Školství 30 Zdravotnictví a sociální zabezpečení 31 Kultura 33 Cestovní ruch 34 Ekologie 35 Sport 36 MÍSTNÍ EKONOMIKA Zaměstnavatelé ve městě Valašské Klobouky 37 SPRÁVA MĚSTA Zadluženost města a vývoj dluhové služby 38 Výhled příjmů a výdajů města do roku 2005 38 3 POSTAVENÍ MĚSTA Valašské Klobouky leží v jihovýchodní části Zlínského kraje, v mikroregionu V RÁMCI ČESKÉ Jižní Valašsko. Město spadá do severní části Chráněné krajinné oblasti Bílé REPUBLIKY Karpaty. Valašské Klobouky jsou město s pověřenými úřady třetího stupně. Počet obyvatel je v současnosti 5191. Valašské Klobouky leží cca 10 km od státní hranice se Slovenskou republikou, mezi Horní Lidčí, což je poslední významný železniční uzel před hranicí se Slovenskou republikou ve Zlínském kraji a také železniční hraniční přechod, a od automobilového hraničního přechodu Brumov-Bylnice/Horné Srnie. S Horní Lidčí jsou Valašské Klobouky spojeny jak železniční tratí s pravidelným vlakovým spojením, tak silnicí první třídy I/57, která z Valašských Klobouk pokračuje do automobilového hraničního přechodu Brumova-Bylnice/Horné Srnie. -

Komentář Ke Statistickému Výkazu Regionálních Funkcí Za 1
Komentář ke statistickému výkazu regionálních funkcí okresu Zlín za rok 2015 1. PORADENSKÁ A KONZULTAČNÍ ČINNOST Počet obsluhovaných knihoven: 118 Počet poskytnutých konzultací: 526 Počet vykonaných metodických návštěv: 165 Z celkového počtu konzultací a metodických návštěv (691) bylo jednáno se starosty: 120 Vykazované číselné údaje zahrnují metodické návštěvy a konzultace v jednotlivých knihovnách, které byly zaměřeny na organizační záležitosti týkající se plnění knihovnických standardů, statistiky knihovnických činností, evidence a zpracování knihovních fondů zakoupených z finančních prostředků provozovatelů knihoven, revize a aktualizace knihovních fondů, práce s výměnným fondem, žádosti starostů o pomoc při svozu/rozvozu výměnných souborů, vykazování výkonu regionálních funkcí, vytvoření elektronické adresy knihovny, webových stránek knihoven. Během roku 2015 byly provedeny estetické úpravy, případně stěhování v těchto knihovnách: Bohuslavice nad Vláří (stěhování do nových prostor, kompletní vybavení novým nábytkem), Bohuslavice u Zlína (nový PC s tiskárnou, knihovna vymalována, nový koberec a záclona, nově natřené regály, dětský koutek s hračkami, nové uspořádání regálů), Březnice (nový PC a tiskárna, nové WC v knihovně a bezbariérový přístup), Drnovice (dvě nové skříňky na knihy a časopisy), Haluzice (nový PC), Hostišová (úprava interiéru, nové rozřaďovače, změna názvu), Hřivínův Újezd (nový PC a tiskárna), Kašava (přestěhována do nových prostor, využity vyřazené regály z Lukova), Křekov (nové hodiny, rychlovarná konvice, barevná -

Autorizatii 2002-2021
MINISTERUL AFACERILOR INTERNE DEPARTAMENTUL PENTRU SITUAŢII DE URGENŢĂ INSPECTORATUL GENERAL PENTRU SITUAŢII DE URGENŢĂ INSPECTORATUL PENTRU SITUAŢII DE URGENŢĂ „DROBETA” AL JUDEŢULUI MEHEDINŢI EVIDENŢA AUTORIZAŢIILOR DE SECURITATE LA INCENDIU EMISE ÎN PERIOADA 2002–2021 ÎN JUDEŢUL MEHEDINŢI Număr Nr. autorizaţie de Denumirea titularului Adresa/Denumirea construcţiei pentru care a fost emisă autorizaţia crt. securitate la autorizaţiei de securitate la incendiu incendiu 2002 MUN. DROBETA TURNU SEVERIN, B-DUL REVOLUTIEI 16-22 DECEMBRIE 1989, 1. 604071 SC PETROM SA NR.21G BENZINARIE NR. 7 COM. ŞIMIAN 2. 604072 BUZGURE CONSTANTIN DANIEL MOTEL BALOTA COM. STANGACEAUA, CALEA CRAIOVEI, KM. 281 3. 604073 STEFANOIU EUGEN STATIE DISTRIBUTIE CARBURANTI DROBETA TURNU SEVERIN, STR. TRAIAN, NR. 47A 4. 604075 GHEORGHICEANU FLORIN MAGAZIN MUN. DROBETA TURNU SEVERIN, STR. NUFERILOR 5. 604076 MLADIN LAVINIA ADELAIDA AMENAJARE SPATIU COMERCIAL INTR-UN IMOBIL DE LOCUIT MUN. DROBETA TURNU SEVERIN, B-DUL I.C. BRATIANU COLT CU B-DUL MIHAI 6. 604077 SC OMV ROMANIA SRL VITEAZU STATIE PECO MUN. DROBETA TURNU SEVERIN, STR. AEROPORTULUI, NR. 3 7. 604078 SC ABC SYSTEMS SRL STATIE IMBUTELIERE OXIGEN COM. OPRISOR 8. 604079 BALACEANU VALTER IOANIDE STATIE PECO TRANSPORTABILA MUN. DROBETA TURNU SEVERIN, STR. I.L. CARAGIALE, NR. 39 9. 604080 A.F. TROPICAL 2002 SPATII CAZARE 1 MUN. DROBETA TURNU SEVERIN, INTERSECTIA B-DUL MIHAI VITEAZU CU STR. 10. 604081 SC FLORA SERCOM SA PRIVIGHETORILOR STATIE PECO TRANSPORTABILA SC FONTEGAS ROCCADASPIDE COM. SIMIAN 11. 604082 ITALIA SRL DEPOZIT DE GAZ BUTAN MUN. DROBETA TURNU SEVERIN, STR. BANOVITEI, NR. 46 12. 604083 SC BERE SPIRT SA INSTALATIE DISTILARE-RAFINARE 2003 MUN. DROBETA TURNU SEVERIN, STR. -

Official Journal L129
Official Journal L 129 of the European Union ★ ★ ★ ★ ★ ★ ★ ★ ★ ★ ★ ★ Volume 62 English edition Legislation 17 May 2019 Contents II Non-legislative acts REGULATIONS ★ Commission Implementing Regulation (EU) 2019/791 of 16 May 2019 amending for the 302nd time Council Regulation (EC) No 881/2002 imposing certain specific restrictive measures directed against certain persons and entities associated with the ISIL (Da'esh) and Al-Qaida organisations ..................................................................................................... 1 DECISIONS ★ Council Decision (EU) 2019/792 of 13 May 2019 entrusting to the European Commission — the Office for the Administration and Payment of Individual Entitlements (PMO) — the exercise of certain powers conferred on the appointing authority and the authority empowered to conclude contracts of employment ............................................................... 3 ★ Commission Implementing Decision (EU) 2019/793 of 16 May 2019 amending the Annex to Implementing Decision 2014/709/EU concerning animal health control measures relating to African swine fever in certain Member States (notified under document C(2019) 3797) (1) ............... 5 RECOMMENDATIONS ★ Commission Recommendation (EU) 2019/794 of 15 May 2019 on a coordinated control plan with a view to establishing the prevalence of certain substances migrating from materials and articles intended to come into contact with food (notified under document C(2019) 3519) (1) .......... 37 (1) Text with EEA relevance. (Continued overleaf) -

1552058338 Narizeni Statni Ve
e. 1. SVSlzo 1 91029434-2 ilt il]flillilfi ilililfl ilt iltffi ilililIIil svsres743b1e07 NARIZENI sTalui VETERINAnTi SPRAVY cl. t Krajsk6 veterindrnl spr6va St6tni veterinirni sprdvy pro Zlinskli kraj, jako spr6vni orgdn piislu5nf podle $ 47 odst. 4 a7 a podle $ 49 odst, 1 pism. c) zAkona 6. 166/1999 Sb., o veterinArnlp66i a o zm6n6 nEkterlich souvisejicfch zdkon&, ve zndni pozd6j5ich piedpisrl (d6le jen ,,veterin6rni zAkon"), v souladu s $ 17 odst. 1 pism. a) a b) veterin6rniho zdkona timto ukonduje mimoiidn6 veterin6rni opatieni vyhli5eni k zamezenI Siieni nebezpe6n6 n6kazy-moru vdeliho plodu v fzemnim obvodu Zlinsk6ho kraje 1. Nai[zenlm St6tni veterin6rnI spr6vy 6. j. SVS/20171093613-Z ze dne 4. 8. 2017, pro ochrann6 p6smo ziizen5 kolem ohniska v obci Trnava, okres Zlin, v katastr6lnlch fzemlch obc( v okrese Zlin: Byezovd u Zlina, De5nd u Zlina, DrZkov6, Hrobice na Morav€, Chrast65ov, Jasenn5 na Morav6, KaSava, Lutonina, Neubuz, Ostrata, Podkopnd Lhota, Rakovd, SluSovice, Trnava u Zlina, Velikovd, Vizovice, Vldkovd a V5emina, v okrese Vsetin: Ho5t'6lkov6 a Lipt6l, 2. NaiizenimStdtni veterin6rni sprdvy6. j. SVS/20171093636-2ze dne4.8.2017, proochrann6 p6smo ziizenl kolem ohniska v obci Kladn6 Zilin, okres Zlin,v katastr6lnich Uzemich obci v okrese Zlin: Biskupice u Luhadovic, Dolni Lhota u Luhadovic, Hornl Lhota u Luha6ovic, Kladnd Zilin, Ludkovice, Luhadovice, Nev5ov6, Petr&vka u Slavidina, Podhradi u Luhadovic, Polichno, Pozlovice, Rudimov, Retechov, Sehradice a Slavidin, v okrese Uhersk6 Hradi5t6: Bojkovice, Pie6kovice, Rudice, Ujezdec u Luha6ovic a Z6horovice. etz Toto naiizeni Stdtni veterindrnI sprdvy nablivri podle $ 76 odst. 3 pism. a) veterin6rniho zdkona platnosti a Udinnosti dnem jeho vyhl65eni; za den jeho vyhl65enI se povaZuje prvni den jeho vyv6Seni na Uiedni desce Krajsk6ho 0iadu Zlinskeho kraje. -

Kandidátní Listiny Republikánské Strany Pro Volby Do Obecních
Kandidátní listiny republikánské strany pro volby do obecních zastupitelstev v politickém okrese Uherském Brodě 1938 Zkratky: OZ=obecnízastupitelstvo Obec:BÁNOV Rokvolby: 1938 KandidátnílistinaRepublikánské stranyzemědělskéhoamalorolnickéholiduaDorostu o ŠvehlíkLudvík,malorolníka pokladníkspoř.azál.spol.,Bánov307členOZ o ZálešákMaxmilián,kolář,Bánov342 o BěhalJaroslav,nájemcestatku,BánovOrdějov80 KandidátnílistinaRepublikánské stranyzemědělskéhoamalorolnickéholidu o ZálešákJan,rolník,Bánov14členOZ o ŠvehlíkLudvík.rolník,Bánov274členOZ o ÚšelaFerdinand,rolník,Bánov17 o MandíkKarel,rolník,Bánov1 o PíškaAugustin,rolník,Bánov78 o SobotkaJan,rolník,Bánov80 o ŠvehlíkJan,rolník,Bánov233 o HavelJiří,rolník,Bánov108 o ČubíkJosef,rolník,Bánov68 o HaurlandTomáš,rolník,Bánov20 o MahdalFrantišek,rolník,Bánov29 Kandidátní listina Republikánské strany zemědělského a malorolnického lidu - Odborovájednotarepublikánských zaměstnanců o BublíkMartin,Bánov314členOZ o KočicaFrantišek,Bánov26 o JurnýVáclav,Bánov37 o MahdalAlois,Bánov335 o PetrůjVáclav,Bánov75 o ZálešákMichal,Bánov182 o JankůjVáclav,Bánov422 o ZálešákFrantišek,Bánov441 o KočicaStanislav,Bánov200 o HauerlandRudolf,Bánov294 o MadajJan,Bánov121 o Hnilička Martin,Bánov66 Kandidátní listina Republikánské strany zemědělského a malorolnického lidu Občansko-rolnická o MahdalFlorián,rolník,Bánov79členOZaradní o KorčekJosef,rolník,Bánov104členOZ o NovákTomáš,rolník,Bánov5 o BeníčekJan.rolník,Bánov49 o VystrčilFrantišek,rolník,Bánov110 o ZálešákŠimon,rolník,Bánov77 Kandidátní listina Republikánské strany -

Rešerše Literatury
UNIVERZITA PALACKÉHO V OLOMOUCI Přírodovědecká fakulta Katedra geografie Dopravní obslužnost obcí v rámci SO ORP Zlín Bakalářská práce Václav Polášek Vedoucí práce: Mgr. Miloslav Šerý Olomouc 2012 Prohlašuji, že jsem zadanou bakalářskou práci vypracoval samostatně pod vedením pana Mgr. Miloslava Šerého a že jsem uvedl veškerou použitou literaturu a další zdroje. V Olomouci dne: Podpis: Na tomto místě bych chtěl poděkovat panu Mgr. Miloslavu Šerému za jeho čas, trpělivost, odborné rady a cenné připomínky, které mi pomohly při zpracování této bakalářské práce. OBSAH 1. ÚVOD.................................... .............................................. 7 2. CÍLE PRÁCE ........................................................................... 9 3. METODIKA PRÁCE .................................................................. 10 4. REŠERŠE LITERATURY .............................................................. 15 5. CHARAKTERISTIKA VYMEZENÉHO ÚZEMÍ ........................................ 18 6. DOPRAVNÍ POLOHA SO ORP ZLÍN ................................................. 20 7. DOPRAVNÍ POLOHA OBCÍ .......................................................... 23 8. DOPRAVNÍ OBSLUŢNOST V RÁMCI SO ORP ZLÍN ................................ 26 8.1 Stručný vývoj veřejné dopravy na Zlínsku od roku 1948 ............ 26 8.2 Dopravní podniky v současnosti ........................................ 27 8.3 Dálkoví dopravci působící v SO ORP Zlín .............................. 28 8.4 Integrovaný dopravní systém Zlínské kraje ..........................